Abstract
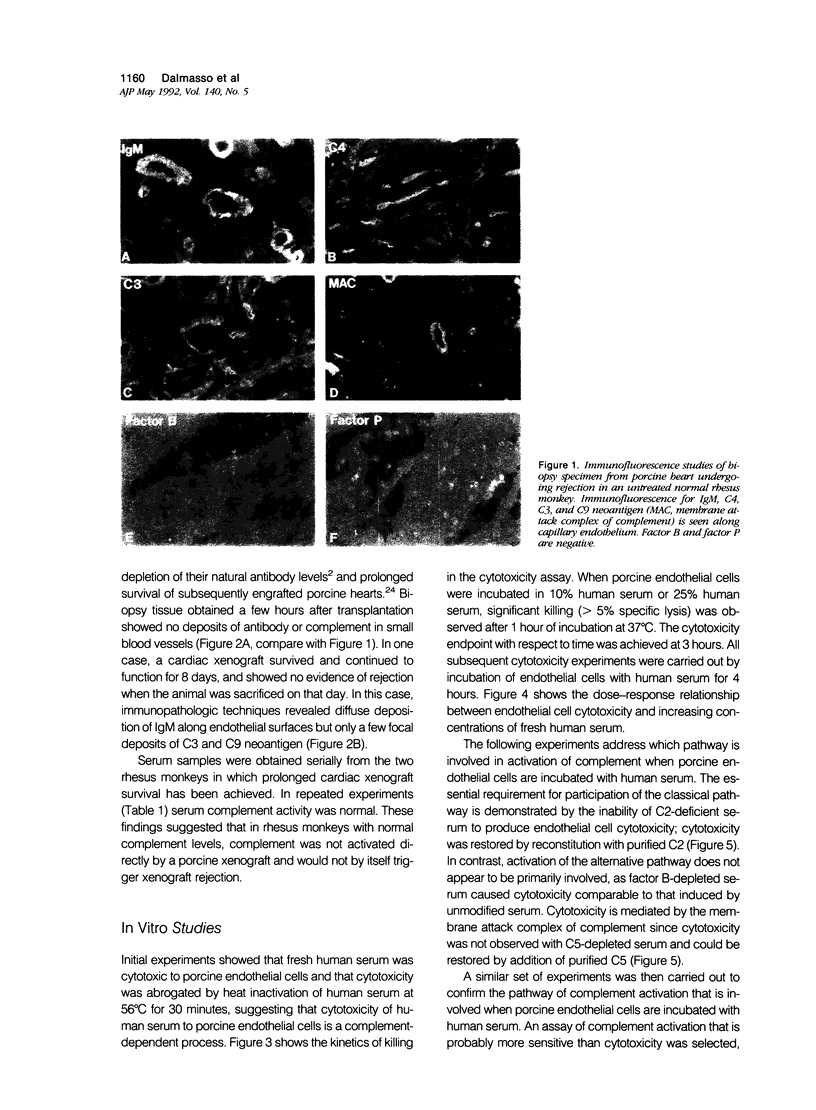
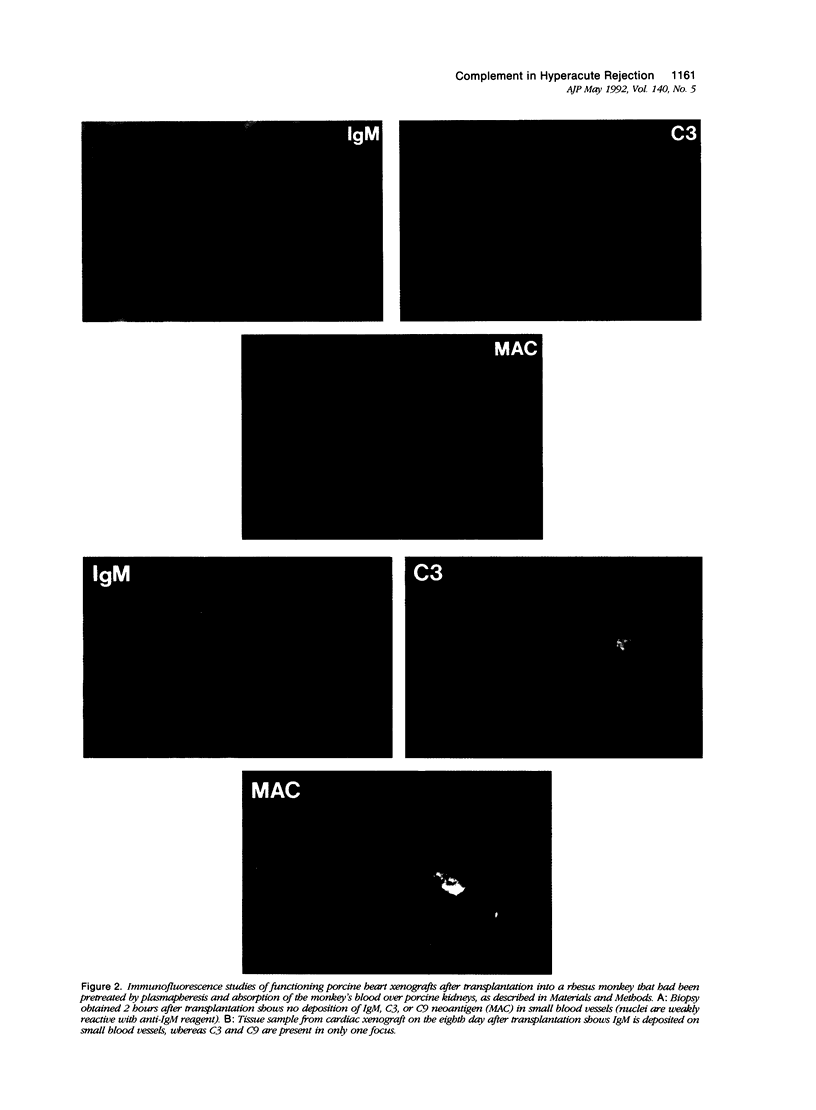
The authors investigated the importance of natural antibody and complement in the pathogenesis of hyperacute xenograft rejection using in vivo and in vitro pig to primate models. Studies were carried out in rhesus monkeys transplanted with a pig heart or kidney in which hyperacute rejection was observed within a few hours. The rejected organs showed deposits of IgM, C3, C4, C5, and C9 neoantigen along small blood vessels, but few deposits of factors B and P. Removal of anti-endothelial cell "natural" antibodies by plasmapheresis, immunoabsorption, and immunosuppression techniques resulted in marked prolongation of the survival of a subsequently transplanted heart, even when complement levels were within the normal range. Thus, complement, in the absence of natural antibodies, did not initiate hyperacute rejection in this species combination. The requirements for complement activation in human serum to cause cytotoxicity of porcine endothelial cells were then evaluated. Cytotoxicity was abrogated by depleting human serum of IgM, C2, or C5, but not of factor B. Restoration of the effect of serum on endothelial cells was achieved by reconstitution of the respective depleted sera with purified IgM or with the corresponding complement proteins, indicating that IgM and the classical, but not the alternative, pathway of complement, were involved. Identical conclusions were drawn from experiments to ascertain the requirements for complement activation in human serum to mediate binding of iC3b to porcine endothelial cells. The authors conclude that in a pig to primate xenograft complement does not directly initiate injury to the graft but rather requires activation by bound xenoreactive natural antibodies; IgM antibodies directed against endothelial cells activate the classical complement pathway, which then contributes to endothelial cell activation and subsequent events characteristic of hyperacute rejection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Rosengard B. R., Hutchins G. M., Hall T. S., Baumgartner W. A., Borkon A. M., Reitz B. A. Effects of cyclosporine, aspirin, and cobra venom factor on discordant cardiac xenograft survival in rats. Transplant Proc. 1987 Feb;19(1 Pt 2):1145–1148. [PubMed] [Google Scholar]
- Auchincloss H., Jr Xenogeneic transplantation. A review. Transplantation. 1988 Jul;46(1):1–20. doi: 10.1097/00007890-198807000-00001. [DOI] [PubMed] [Google Scholar]
- Cooper D. K., Human P. A., Lexer G., Rose A. G., Rees J., Keraan M., Du Toit E. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988 May-Jun;7(3):238–246. [PubMed] [Google Scholar]
- Dalmasso A. P. Complement in the pathophysiology and diagnosis of human diseases. Crit Rev Clin Lab Sci. 1986;24(2):123–183. doi: 10.3109/10408368609110272. [DOI] [PubMed] [Google Scholar]
- Dalmasso A. P., Vercellotti G. M., Platt J. L., Bach F. H. Inhibition of complement-mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation. 1991 Sep;52(3):530–533. doi: 10.1097/00007890-199109000-00029. [DOI] [PubMed] [Google Scholar]
- Edwards J. Complement activation by xenogeneic red blood cells. Transplantation. 1981 Mar;31(3):226–227. [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983 Aug;72(2):560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischel R. J., Bolman R. M., 3rd, Platt J. L., Najarian J. S., Bach F. H., Matas A. J. Removal of IgM anti-endothelial antibodies results in prolonged cardiac xenograft survival. Transplant Proc. 1990 Jun;22(3):1077–1078. [PubMed] [Google Scholar]
- Fischel R. J., Platt J. L., Matas A. J., Perry E., Dalmasso A. P., Manivel C., Najarian J. S., Bach F. H., Bolman R. M., 3rd Prolonged survival of a discordant cardiac xenograft in a rhesus monkey. Transplant Proc. 1991 Feb;23(1 Pt 1):589–590. [PubMed] [Google Scholar]
- Gewurz H., Clark D. S., Cooper M. D., Varco R. L., Good R. A. Effect of cobra venom-induced inhibition of complement activity on allograft and xenograft rejection reactions. Transplantation. 1967 Sep 5;5(5):1296–1303. doi: 10.1097/00007890-196709000-00008. [DOI] [PubMed] [Google Scholar]
- Hamilton K. K., Hattori R., Esmon C. T., Sims P. J. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem. 1990 Mar 5;265(7):3809–3814. [PubMed] [Google Scholar]
- Hammer C. Isohemagglutinins and preformed natural antibodies in xenogeneic organ transplantation. Transplant Proc. 1987 Dec;19(6):4443–4447. [PubMed] [Google Scholar]
- Henry M. L., Han L. K., Orosz C. G., Sedmak D., Ferguson R. M. Modification of xenograft hyperacute rejection via xenoantibody depletion. Transplant Proc. 1990 Jun;22(3):1081–1082. [PubMed] [Google Scholar]
- Hourcade D., Holers V. M., Atkinson J. P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- Johnston P. S., Lim S. M., Wang M. W., Wright L., White D. J. Hyperacute rejection of xenografts in the complete absence of antibody. Transplant Proc. 1991 Feb;23(1 Pt 1):877–879. [PubMed] [Google Scholar]
- Kemp E., Steinbrüchel D., Starklint H., Larsen S., Henriksen I., Dieperink H. Renal xenograft rejection: prolonging effect of captopril, ACE-inhibitors, prostacyclin, and cobra venom factor. Transplant Proc. 1987 Dec;19(6):4471–4474. [PubMed] [Google Scholar]
- Mahowald M. L., Dalmasso A. P., Petzel R. A., Yunis E. J. Linkage relationship of C2 deficiency, HLA and glyoxalase I loci. Vox Sang. 1979;37(6):321–328. doi: 10.1111/j.1423-0410.1979.tb02311.x. [DOI] [PubMed] [Google Scholar]
- Marks R. M., Todd R. F., 3rd, Ward P. A. Rapid induction of neutrophil-endothelial adhesion by endothelial complement fixation. Nature. 1989 May 25;339(6222):314–317. doi: 10.1038/339314a0. [DOI] [PubMed] [Google Scholar]
- Mejĩa-Laguna J. E., Martĩnez-Palomo A., Biro C. E., Chãvez B., Lõpez-Soriano F., Carcĩa-Cornejo M. Morphologic study of the participation of the complement system in hyperacute rejection of renal xenotransplants. Am J Pathol. 1972 Oct;69(1):71–78. [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S., Hirose H., Shirakura R., Naka Y., Nakata S., Kawashima Y., Seya T., Matsumoto M., Uenaka A., Kitamura H. The mechanism of discordant xenograft rejection. Transplantation. 1988 Dec;46(6):825–830. doi: 10.1097/00007890-198812000-00007. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Zalman L. S., Chiu F. J., Jung G., Martin D. E. Molecular mechanisms of cytotoxicity: comparison of complement and killer lymphocytes. J Rheumatol Suppl. 1987 Jun;14 (Suppl 13):28–34. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan C. C., Robitaille P., Pinto-Blonde M., Chartrand C. Delayed rejection of cardiac xenografts in C6-deficient rabbits. Immunology. 1979 Oct;38(2):245–248. [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. The alternative pathway of complement. Springer Semin Immunopathol. 1984;7(2-3):163–192. doi: 10.1007/BF01893019. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Fischel R. J., Matas A. J., Reif S. A., Bolman R. M., Bach F. H. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991 Aug;52(2):214–220. doi: 10.1097/00007890-199108000-00006. [DOI] [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Interstitial mononuclear cell populations in renal graft rejection. Identification by monoclonal antibodies in tissue sections. J Exp Med. 1982 Jan 1;155(1):17–30. doi: 10.1084/jem.155.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Turman M. A., Noreen H. J., Fischel R. J., Bolman R. M., 3rd, Bach F. H. An ELISA assay for xenoreactive natural antibodies. Transplantation. 1990 May;49(5):1000–1001. doi: 10.1097/00007890-199005000-00033. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Vercellotti G. M., Dalmasso A. P., Matas A. J., Bolman R. M., Najarian J. S., Bach F. H. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990 Dec;11(12):450–457. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Vercellotti G. M., Lindman B. J., Oegema T. R., Jr, Bach F. H., Dalmasso A. P. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990 Apr 1;171(4):1363–1368. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Snyder G. B., Ballesteros E., Zarco R. M., Linn B. S. Prolongation of renal xenografts by complement suppression. Surg Forum. 1966;17:478–480. [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Fifth component of human complement: purification from plasma and polypeptide chain structure. Biochemistry. 1979 Apr 17;18(8):1490–1497. doi: 10.1021/bi00575a016. [DOI] [PubMed] [Google Scholar]
- Turman M. A., Casali P., Notkins A. L., Bach F. H., Platt J. L. Polyreactivity and antigen specificity of human xenoreactive monoclonal and serum natural antibodies. Transplantation. 1991 Oct;52(4):710–717. doi: 10.1097/00007890-199110000-00024. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Platt J. L., Bach F. H., Dalmasso A. P. Neutrophil adhesion to xenogeneic endothelium via iC3b. J Immunol. 1991 Jan 15;146(2):730–734. [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M. Clq: rapid purification method for preparation of monospecific antisera and for biochemical studies. J Immunol. 1971 Feb;106(2):304–313. [PubMed] [Google Scholar]
- Zhow X. J., Niesen N., Pawlowski I., Biesecker G., Andres G., Brentjens J., Milgram F. Prolongation of survival of discordant kidney xenografts by C6 deficiency. Transplantation. 1990 Nov;50(5):896–898. [PubMed] [Google Scholar]