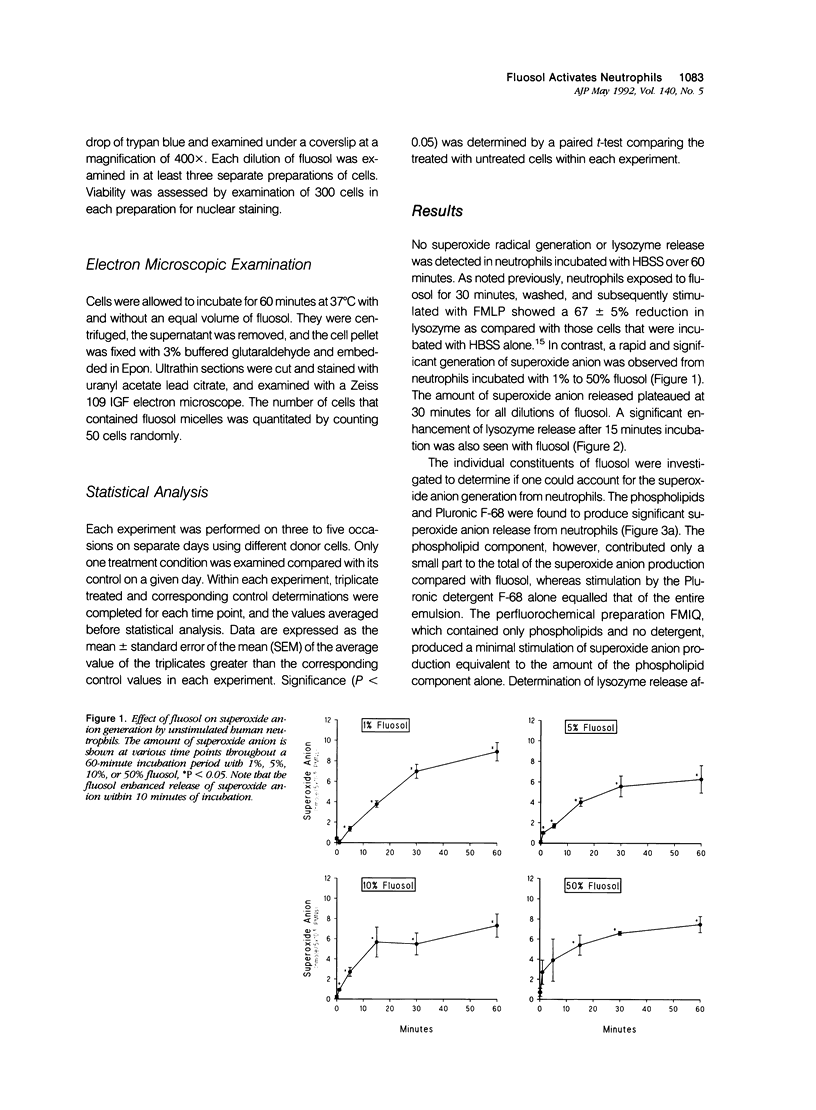
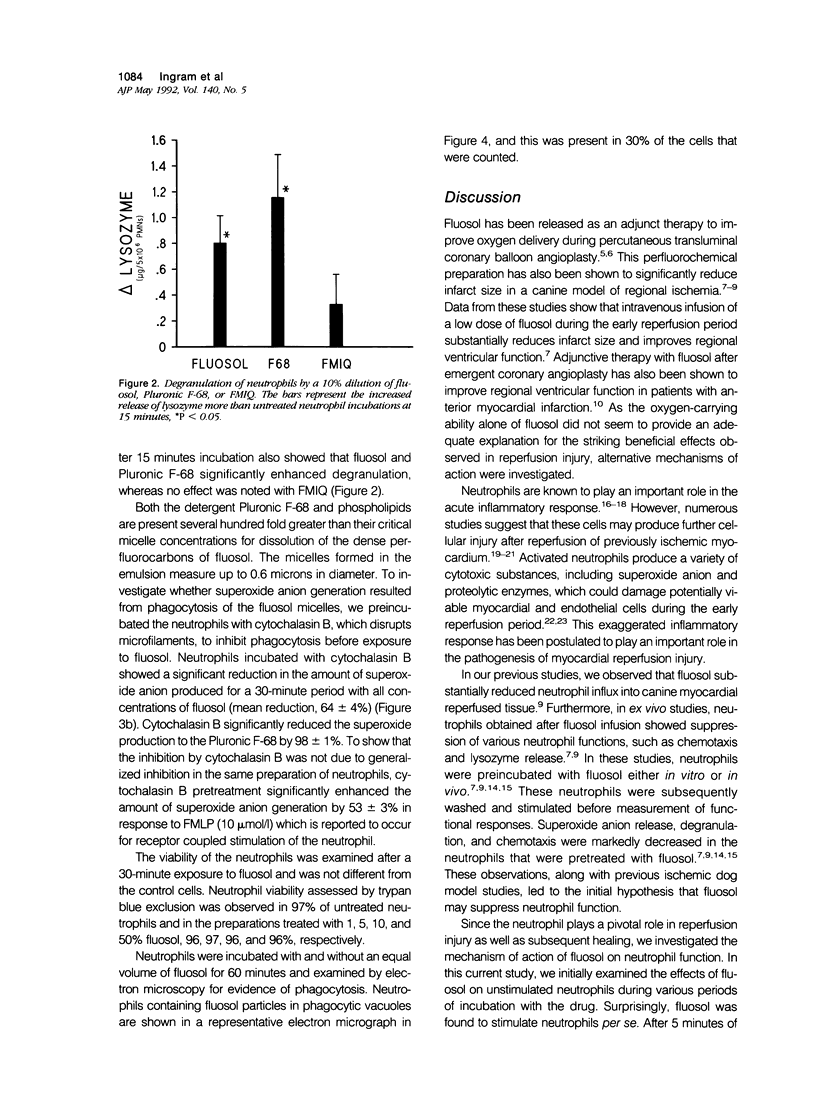
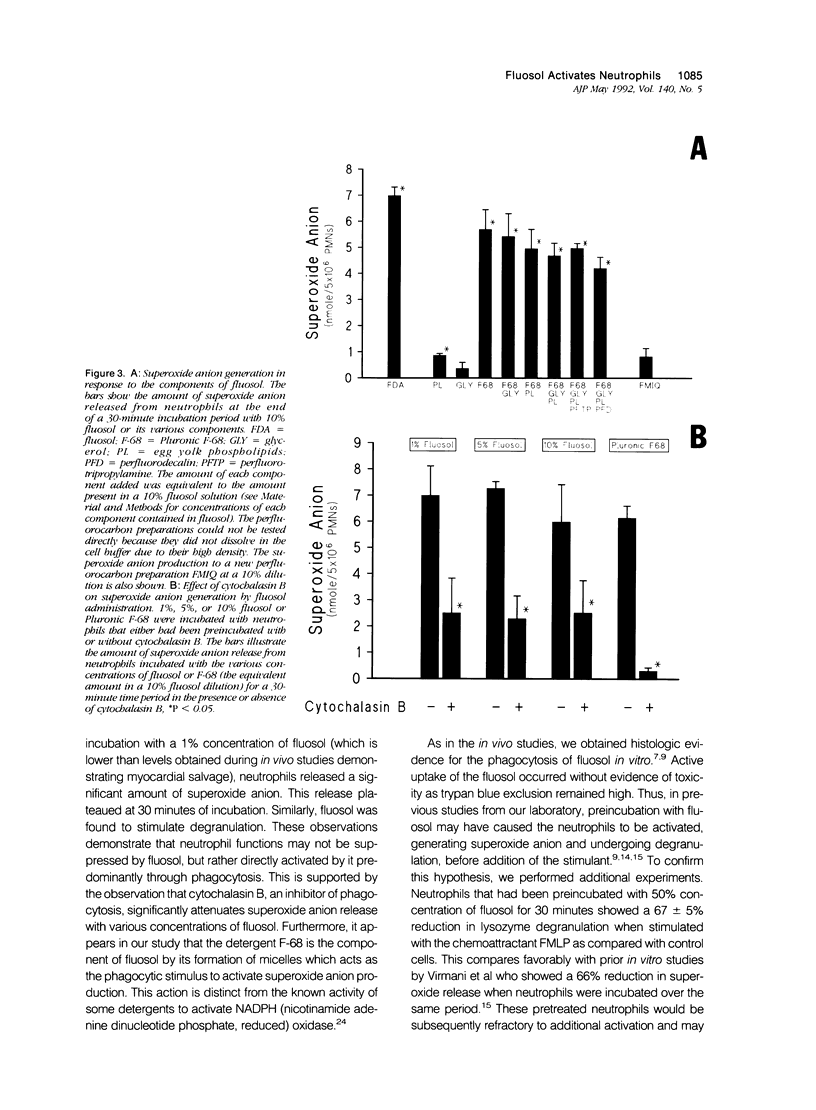
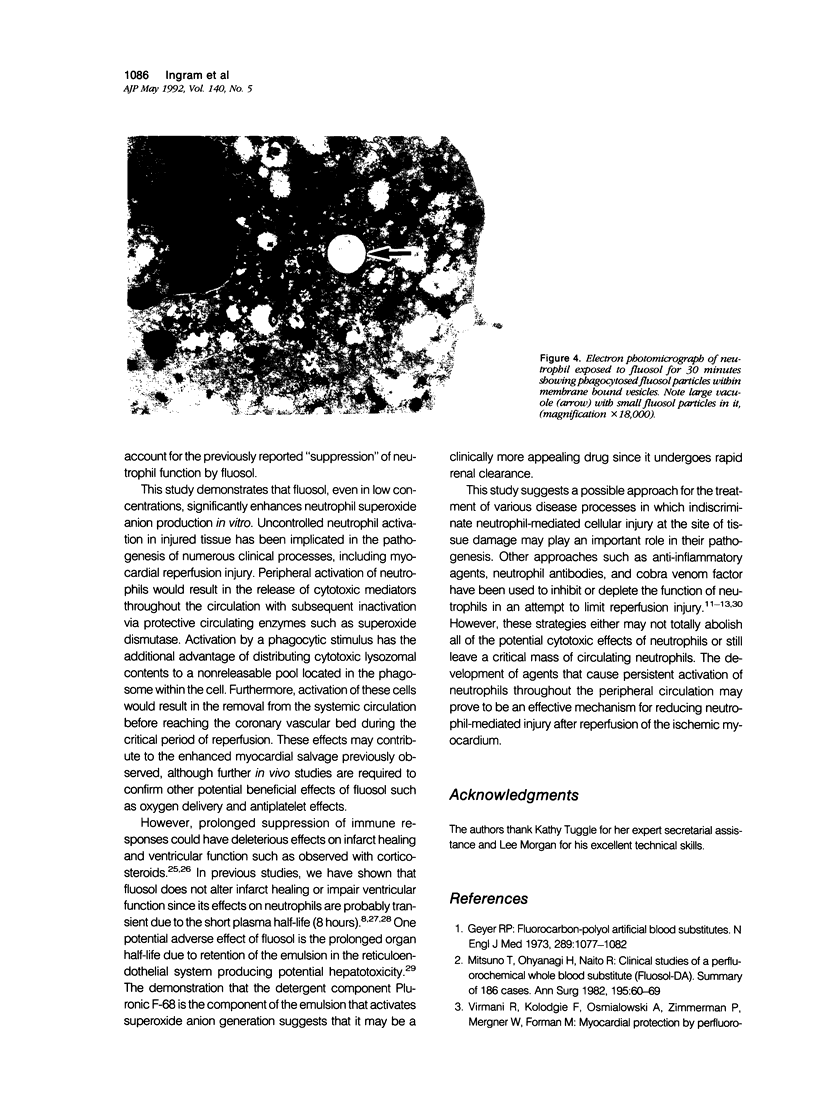
Abstract
Fluosol (Alpha Therapeutic Corporation, Los Angeles, CA) an emulsion of perfluorocarbons with a high oxygen-carrying capacity, was approved as an adjunct to alleviate myocardial ischemia during coronary angioplasty. This drug also significantly enhances myocardial salvage presumably related to an action on the neutrophil. The mechanism by which fluosol and its individual components, including the detergent Pluronic F-68, affected neutrophil function was examined. During the incubation of neutrophils with fluosol, a rapid stimulation of superoxide anion production and degranulation which progressively increased over a 30-minute period was detected. Neutrophils incubated with only Pluronic F-68 produced similar amounts of superoxide anion. Cytochalasin B, an inhibitor of phagocytosis, significantly inhibited this superoxide anion generation. As shown previously, neutrophils incubated with fluosol for 30 minutes and then subsequently stimulated manifested a reduction in lysozyme release as compared with untreated cells. Results of an electron microscopic examination confirmed the cellular uptake of the fluosol within phagocytic vacuoles. Neutrophil viability determined by trypan blue was unaffected after fluosol treatment. These observations show that the fluosol emulsion, primarily through micelles formed by the detergent Pluronic F-68, activates human neutrophils by serving as a phagocytic stimulus, which produces a cell refractory to subsequent stimulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt D. G., Forman M. B., Jones R., Bajaj A. K., Hoover R. L. Prevention of neutrophil-mediated injury to endothelial cells by perfluorochemical. Am J Pathol. 1990 Feb;136(2):451–459. [PMC free article] [PubMed] [Google Scholar]
- Bajaj A. K., Cobb M. A., Virmani R., Gay J. C., Light R. T., Forman M. B. Limitation of myocardial reperfusion injury by intravenous perfluorochemicals. Role of neutrophil activation. Circulation. 1989 Mar;79(3):645–656. doi: 10.1161/01.cir.79.3.645. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985 Nov;76(5):1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain P., Latour J. G., Tran D., de Lorgeril M., Dupras G., Bourassa M. Neutrophil accumulation in experimental myocardial infarcts: relation with extent of injury and effect of reperfusion. Circulation. 1987 May;75(5):1083–1090. doi: 10.1161/01.cir.75.5.1083. [DOI] [PubMed] [Google Scholar]
- Cleman M., Jaffee C. C., Wohlgelernter D. Prevention of ischemia during percutaneous transluminal coronary angioplasty by transcatheter infusion of oxygenated Fluosol DA 20%. Circulation. 1986 Sep;74(3):555–562. doi: 10.1161/01.cir.74.3.555. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide production by digitonin-stimulated guinea pig granulocytes. The effects of N-ethyl maleimide, divalent cations; and glycolytic and mitochondrial inhibitors on the activation of the superoxide generating system. J Clin Invest. 1978 Apr;61(4):1088–1096. doi: 10.1172/JCI109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Polymorphonuclear leukocyte-mediated cell and tissue injury: oxygen metabolites and their relations to human disease. Hum Pathol. 1985 Oct;16(10):973–978. doi: 10.1016/s0046-8177(85)80273-2. [DOI] [PubMed] [Google Scholar]
- Forman M. B., Perry J. M., Wilson B. H., Verani M. S., Kaplan P. R., Shawl F. A., Friesinger G. C. Demonstration of myocardial reperfusion injury in humans: results of a pilot study utilizing acute coronary angioplasty with perfluorochemical in anterior myocardial infarction. J Am Coll Cardiol. 1991 Oct;18(4):911–918. doi: 10.1016/0735-1097(91)90746-v. [DOI] [PubMed] [Google Scholar]
- Forman M. B., Pitarys C. J., 2nd, Vildibill H. D., Lambert T. L., Ingram D. A., Virmani R., Murray J. J. Pharmacologic perturbation of neutrophils by Fluosol results in a sustained reduction in infarct size in the canine model of reperfusion. J Am Coll Cardiol. 1992 Jan;19(1):205–216. doi: 10.1016/0735-1097(92)90074-w. [DOI] [PubMed] [Google Scholar]
- Forman M. B., Puett D. W., Virmani R. Endothelial and myocardial injury during ischemia and reperfusion: pathogenesis and therapeutic implications. J Am Coll Cardiol. 1989 Feb;13(2):450–459. doi: 10.1016/0735-1097(89)90526-3. [DOI] [PubMed] [Google Scholar]
- Forman M. B., Puett D. W., Wilson B. H., Vaughn W. K., Friesinger G. C., Virmani R. Beneficial long-term effect of intracoronary perfluorochemical on infarct size and ventricular function in a canine reperfusion model. J Am Coll Cardiol. 1987 May;9(5):1082–1090. doi: 10.1016/s0735-1097(87)80311-x. [DOI] [PubMed] [Google Scholar]
- Geyer R. P. Fluorocarbon-polyol artificial blood substitutes. N Engl J Med. 1973 Nov 15;289(20):1077–1082. doi: 10.1056/NEJM197311152892008. [DOI] [PubMed] [Google Scholar]
- Kent K. M., Cleman M. W., Cowley M. J., Forman M. B., Jaffe C. C., Kaplan M., King S. B., 3rd, Krucoff M. W., Lassar T., McAuley B. Reduction of myocardial ischemia during percutaneous transluminal coronary angioplasty with oxygenated Fluosol. Am J Cardiol. 1990 Aug 1;66(3):279–284. doi: 10.1016/0002-9149(90)90836-p. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Fishbein M. C., Lew H., Maroko P. R., Braunwald E. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation. 1978 Jan;57(1):56–63. doi: 10.1161/01.cir.57.1.56. [DOI] [PubMed] [Google Scholar]
- Lutz J., Metzenauer P. Effects of potential blood substitutes (perfluorochemicals) on rat liver and spleen. Pflugers Arch. 1980 Sep;387(2):175–181. doi: 10.1007/BF00584269. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Carpenter C. B., Chiariello M., Fishbein M. C., Radvany P., Knostman J. D., Hale S. L. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978 Mar;61(3):661–670. doi: 10.1172/JCI108978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuno T., Ohyanagi H., Naito R. Clinical studies of a perfluorochemical whole blood substitute (Fluosol-DA) Summary of 186 cases. Ann Surg. 1982 Jan;195(1):60–69. doi: 10.1097/00000658-198201001-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane K. M., Salmon J. A., Kraemer R. Leukocyte-derived metabolites of arachidonic acid in ischemia-induced myocardial injury. Fed Proc. 1987 May 15;46(7):2422–2433. [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- SOMMERS H. M., JENNINGS R. B. EXPERIMENTAL ACUTE MYOCARDIAL INFARCTION; HISTOLOGIC AND HISTOCHEMICAL STUDIES OF EARLY MYOCARDIAL INFARCTS INDUCED BY TEMPORARY OR PERMANENT OCCLUSION OF A CORONARY ARTERY. Lab Invest. 1964 Dec;13:1491–1503. [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Mickelson J. K., Fantone J. C., Gallagher K. P., Lee K. A., Tamura Y., Cronin M., Lucchesi B. R. Sustained limitation of myocardial reperfusion injury by a monoclonal antibody that alters leukocyte function. Circulation. 1990 Jan;81(1):226–237. doi: 10.1161/01.cir.81.1.226. [DOI] [PubMed] [Google Scholar]
- Tremper K. K., Friedman A. E., Levine E. M., Lapin R., Camarillo D. The preoperative treatment of severely anemic patients with a perfluorochemical oxygen-transport fluid, Fluosol-DA. N Engl J Med. 1982 Jul 29;307(5):277–283. doi: 10.1056/NEJM198207293070503. [DOI] [PubMed] [Google Scholar]
- Virmani R., Fink L. M., Gunter K., English D. Effect of perfluorochemical blood substitutes on human neutrophil function. Transfusion. 1984 Jul-Aug;24(4):343–347. doi: 10.1046/j.1537-2995.1984.24484275579.x. [DOI] [PubMed] [Google Scholar]
- Virmani R., Osmialowski A. F., Kolodgie F. D., Forman M. B. The effect of perfluorochemical fluosol-DA (20%) on myocardial infarct healing in the rabbit. Am J Cardiovasc Pathol. 1990;3(1):69–80. [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]



