Abstract
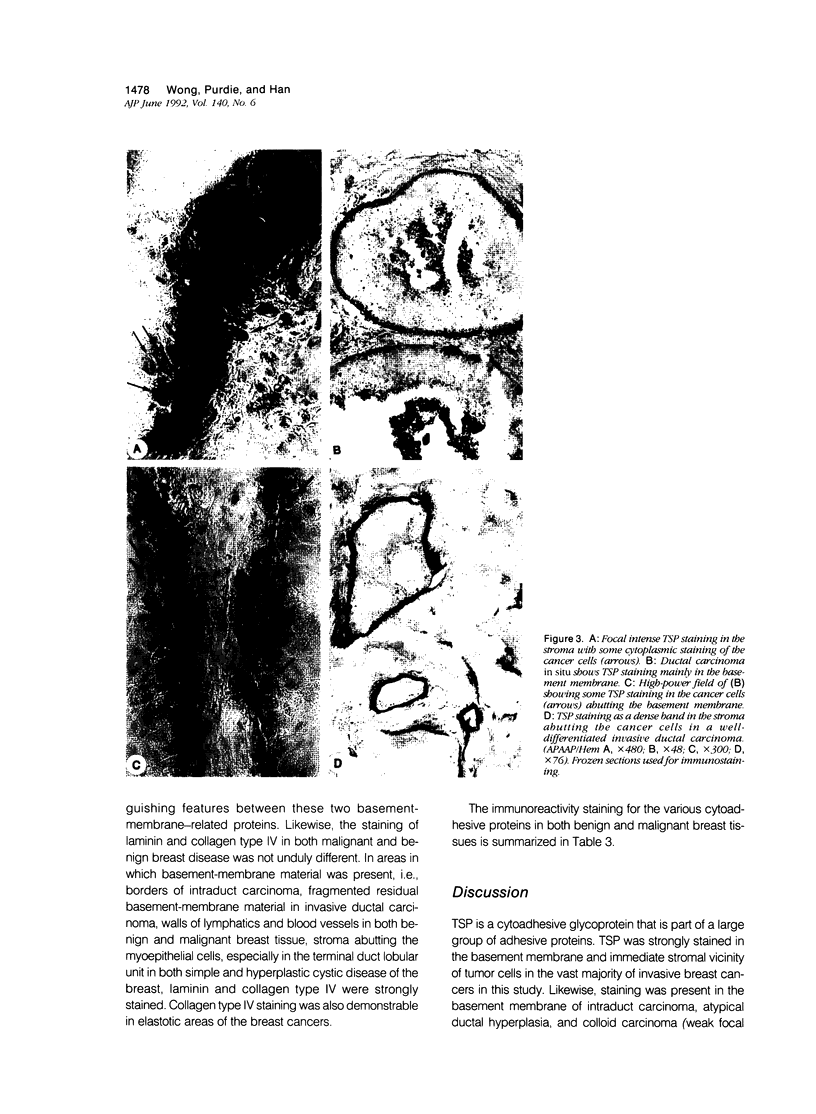
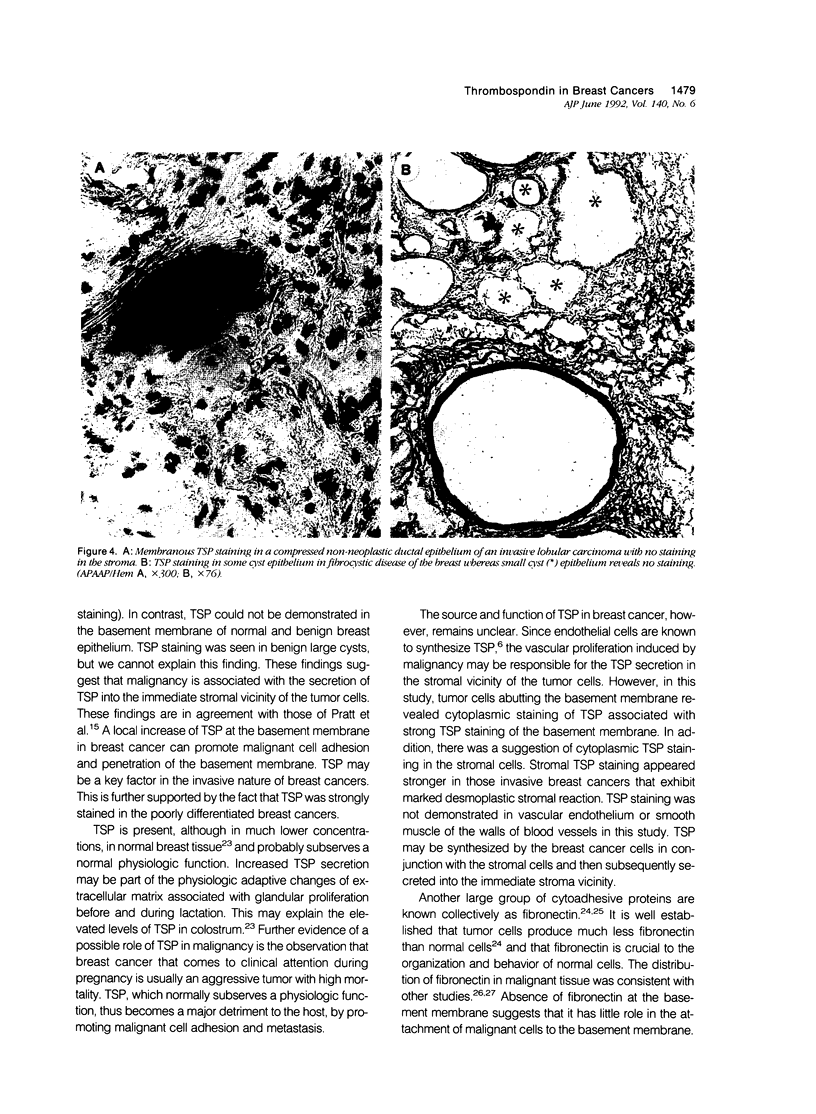
Thrombospondin (TSP), which plays an important hemostatic role, is a 450-kd cytoadhesive protein present in the alpha granules of platelets. In vitro experiments using cultured malignant cells suggest that it may help to mediate malignant cell attachment to extracellular matrix and may be important in cancer invasiveness. The authors studied a group of malignant and benign breast tissues for the expression of TSP and provide evidence that TSP may have a role in tumor invasiveness. Using immunohistochemical techniques, the authors found in 48 fresh specimens of breast carcinoma that TSP stained strongly in the desmoplastic stroma or at the basement membrane associated with the malignant ductal epithelium. Tumor cells abutting these tissues revealed cytoplasmic staining for TSP. Stronger TSP staining was seen in the poorly differentiated tumors. These findings were compared with those of normal and benign breast tissue, which showed no TSP staining apart from reactivity in the large distended cysts of fibrocystic disease and faint staining in the stroma of fibroadenomas. Staining for integrin was positive in lymphocytes of both malignant and benign breast disease and equivocally also in the stromal cells of the breast cancer tissue. Immunoreactivity to TSP in tissues was also compared with that of fibronectin, laminin, and collagen type I, III, and IV. The overall findings suggest that thrombospondin may have a role in the tumor biology of invasiveness.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Nachman R. L. Thrombospondin: phenomenology to function. Prog Hemost Thromb. 1989;9:157–176. [PubMed] [Google Scholar]
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale M. D., Westrick L. G., Mosher D. F. Incorporation of thrombospondin into fibrin clots. J Biol Chem. 1985 Jun 25;260(12):7502–7508. [PubMed] [Google Scholar]
- Clezardin P., Hunter N. R., Lawler J., Dawes J., McGregor J. L. Production, characterization, and use of monoclonal antibodies directed against human blood platelet thrombospondin: immunologic comparison with human endothelial and fibroblast thrombospondins. Semin Thromb Hemost. 1987 Jul;13(3):261–275. doi: 10.1055/s-2007-1003501. [DOI] [PubMed] [Google Scholar]
- Clezardin P., McGregor J. L., Lyon M., Clemetson K. J., Huppert J. Characterization of two murine monoclonal antibodies (P10, P12) directed against different determinants on human blood platelet thrombospondin. Eur J Biochem. 1986 Jan 2;154(1):95–102. doi: 10.1111/j.1432-1033.1986.tb09363.x. [DOI] [PubMed] [Google Scholar]
- D'Ardenne A. J., Barnard N. J. Paucity of fibronectin in invasive lobular carcinoma of breast. J Pathol. 1989 Mar;157(3):219–224. doi: 10.1002/path.1711570308. [DOI] [PubMed] [Google Scholar]
- Davies J. D., Mera S. L. Elastosis in breast carcinoma: II. Association of protease inhibitors with immature elastic fibres. J Pathol. 1987 Dec;153(4):317–324. doi: 10.1002/path.1711530405. [DOI] [PubMed] [Google Scholar]
- Dawes J., Clezardin P., Pratt D. A. Thrombospondin in milk, other breast secretions, and breast tissue. Semin Thromb Hemost. 1987 Jul;13(3):378–384. doi: 10.1055/s-2007-1003514. [DOI] [PubMed] [Google Scholar]
- Elston C. W., Gresham G. A., Rao G. S., Zebro T., Haybittle J. L., Houghton J., Kearney G. The cancer research campaign (King's/Cambridge trial for early breast cancer: clinico-pathological aspects. Br J Cancer. 1982 May;45(5):655–669. doi: 10.1038/bjc.1982.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Fibronectins. Sci Am. 1986 Jun;254(6):42–51. doi: 10.1038/scientificamerican0686-42. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Lahav J., Schwartz M. A., Hynes R. O. Analysis of platelet adhesion with a radioactive chemical crosslinking reagent: interaction of thrombospondin with fibronectin and collagen. Cell. 1982 Nov;31(1):253–262. doi: 10.1016/0092-8674(82)90425-1. [DOI] [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S., Coligan J. E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978 Dec 10;253(23):8609–8616. [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986 May;67(5):1197–1209. [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985 Sep;101(3):1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera S. L., Davies J. D. Elastosis in breast carcinoma: I. Immunohistochemical characterization of elastic fibres. J Pathol. 1987 Feb;151(2):103–110. doi: 10.1002/path.1711510202. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Prasanna H. R. Ca2+-mediated association of glycoprotein G (thrombinsensitive protein, thrombospondin) with human platelets. J Biol Chem. 1980 Dec 25;255(24):11629–11632. [PubMed] [Google Scholar]
- Pratt D. A., Miller W. R., Dawes J. Thrombospondin in malignant and non-malignant breast tissue. Eur J Cancer Clin Oncol. 1989 Feb;25(2):343–350. doi: 10.1016/0277-5379(89)90028-x. [DOI] [PubMed] [Google Scholar]
- Raugi G. J., Mumby S. M., Abbott-Brown D., Bornstein P. Thrombospondin: synthesis and secretion by cells in culture. J Cell Biol. 1982 Oct;95(1):351–354. doi: 10.1083/jcb.95.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Gasic T. B., Rothman V. L., Knudsen K. A., Gasic G. J. Thrombospondin, a potentiator of tumor cell metastasis. Cancer Res. 1987 Aug 1;47(15):4130–4133. [PubMed] [Google Scholar]
- Varani J., Dixit V. M., Fligiel S. E., McKeever P. E., Carey T. E. Thrombospondin-induced attachment and spreading of human squamous carcinoma cells. Exp Cell Res. 1986 Dec;167(2):376–390. doi: 10.1016/0014-4827(86)90178-3. [DOI] [PubMed] [Google Scholar]
- Verhoeven D., Bourgeois N., Buyssens N. Presence of type IV collagen and vitronectin in elastosis. Am J Clin Pathol. 1989 Apr;91(4):505–505. doi: 10.1093/ajcp/91.4.505. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Raugi G. J., Mumby S. M., Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985 Apr;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Wong S. Y., Carrol E. D., Ah-See S. Y., Sewell H. F. Detection of estrogen receptor proteins in breast tumors using an improved APAAP immunohistochemical technique. Cancer. 1988 Nov 15;62(10):2171–2175. doi: 10.1002/1097-0142(19881115)62:10<2171::aid-cncr2820621017>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]
- d'Ardenne A. J. Use of basement membrane markers in tumour diagnosis. J Clin Pathol. 1989 May;42(5):449–457. doi: 10.1136/jcp.42.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]








