Abstract
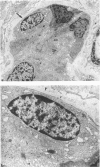
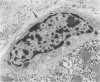
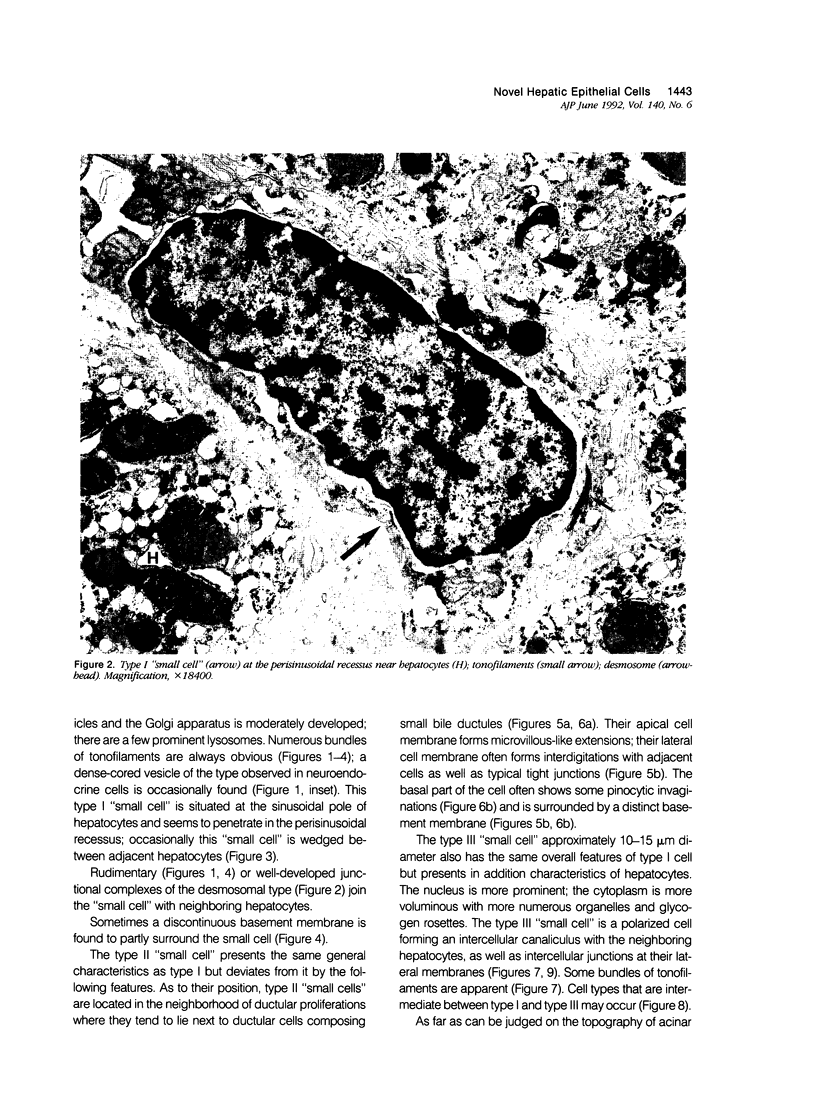
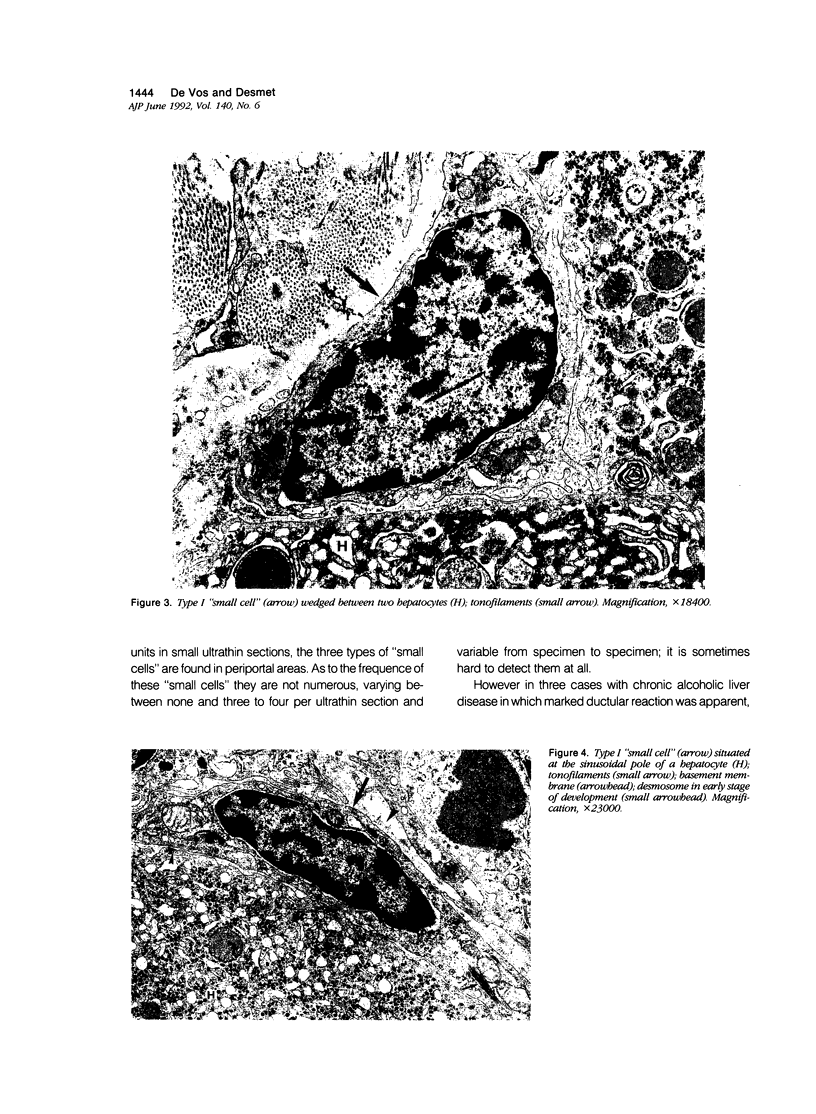
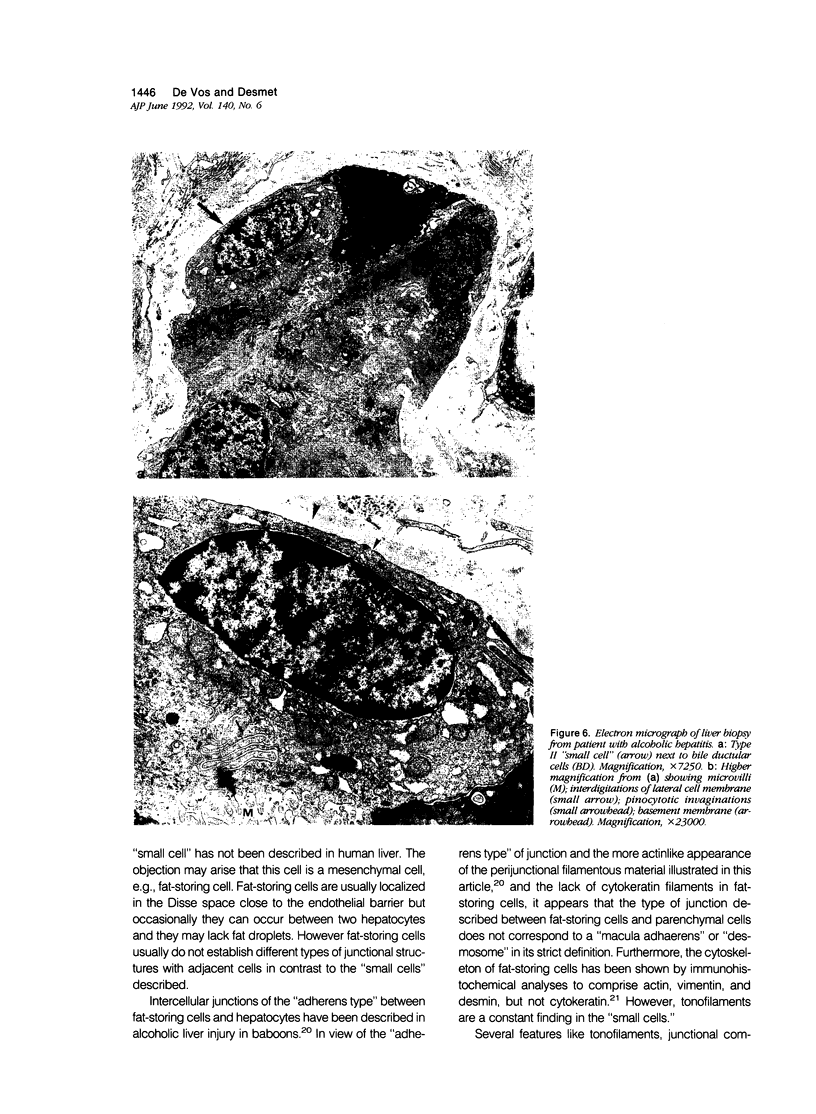
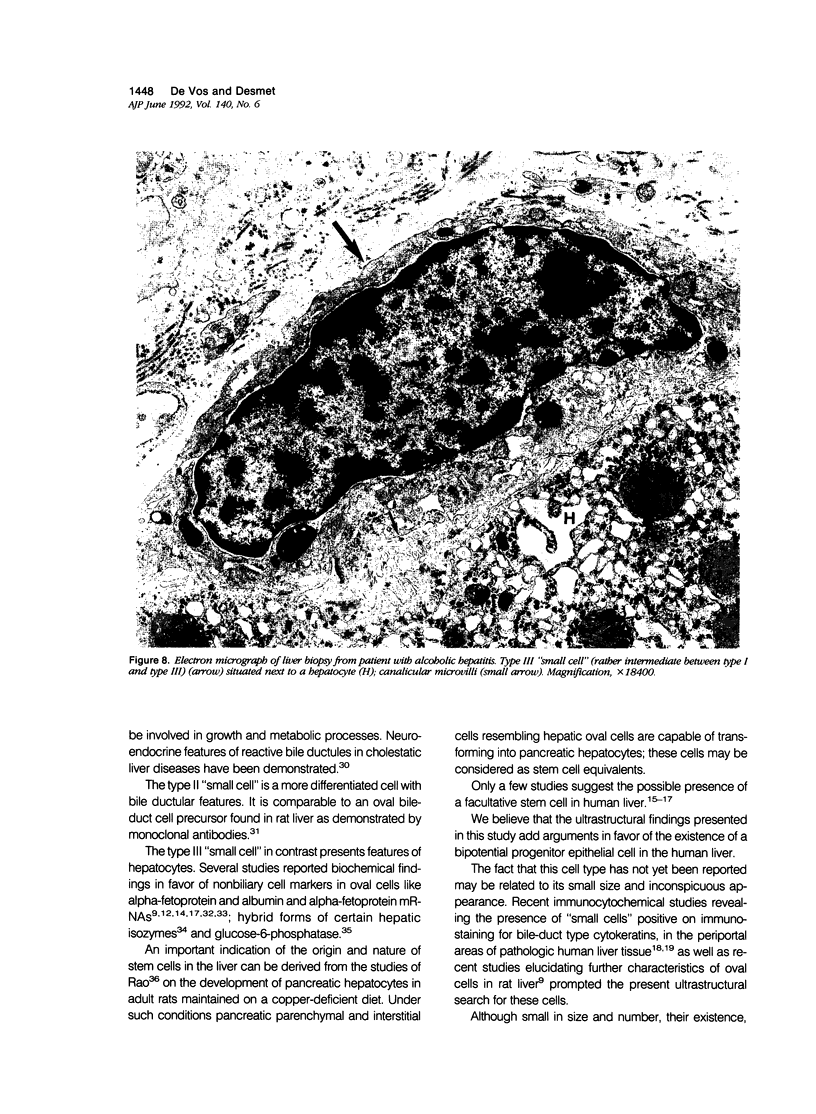
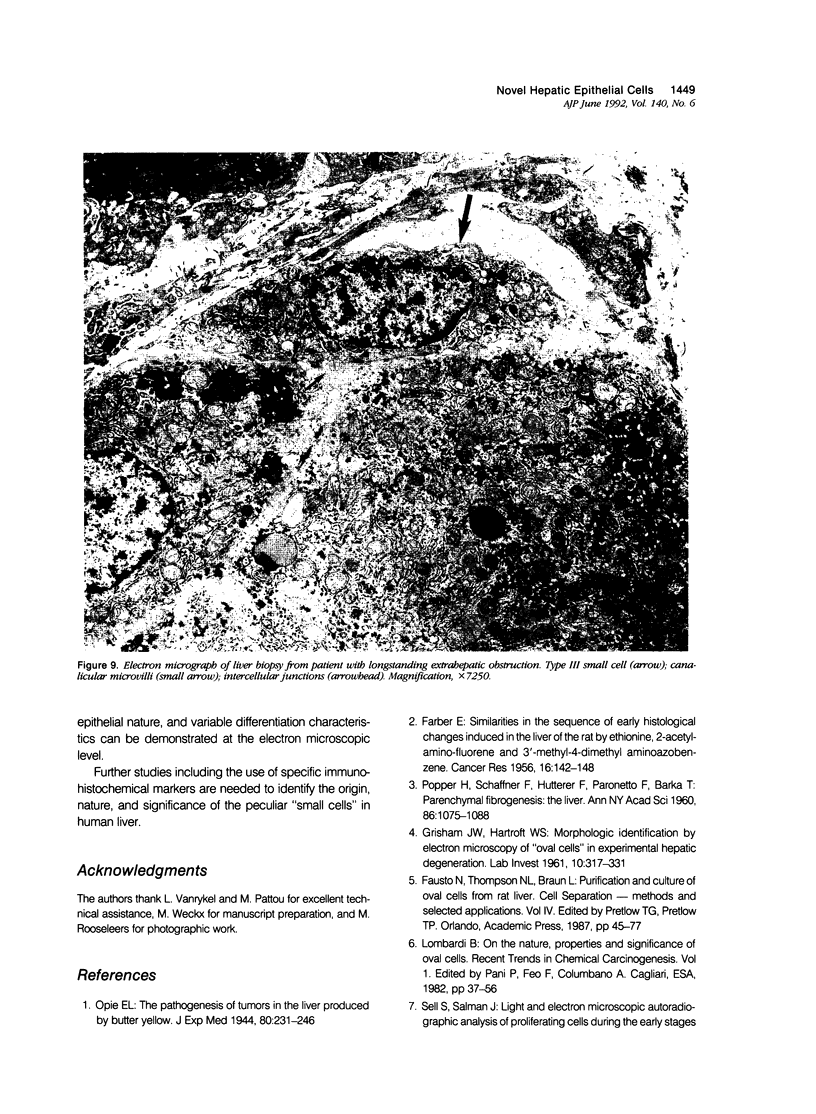
Previous immunohistochemical studies on human liver biopsies with chronic ductular reaction revealed the presence of "small cells" with bile-duct type cytokeratin profile in the periportal area. This study identified similar cells by electron microscopy. The authors studied 13 human liver specimens with various liver diseases, but all characterized by chronic ductular reaction. In all specimens, variable numbers of "small cells" with common epithelial characteristics were identified in the periportal area. They could be classified into three types. Type I cells showed an oval cell shape and oval nucleus, early or established formation of junctional complexes with adjacent cells, a full assortment of cytoplasmic organelles, and bundles of tonofilaments. Type II cells showed features of bile-duct cell differentiation, including lateral interdigitations, apical microvilli, basal pinocytotic vacuoles, and basement membrane formation. In contrast, type III cells displayed additional features indicating hepatocellular differentiation, such as a more prominent nucleus, formation of a hemicanaliculus, and glycogen rosettes. It is concluded that these small cells of epithelial nature display variable differentiation characteristics of either bile-duct type cells or hepatocytes. These findings support the existence of bipotential progenitor epithelial cells in human liver. They may have implications for liver regeneration and carcinogenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber N., Zajicek G., Ariel I. The streaming liver. II. Hepatocyte life history. Liver. 1988 Apr;8(2):80–87. doi: 10.1111/j.1600-0676.1988.tb00972.x. [DOI] [PubMed] [Google Scholar]
- Arber N., Zajicek G. Streaming liver. VI: Streaming intra-hepatic bile ducts. Liver. 1990 Aug;10(4):205–208. doi: 10.1111/j.1600-0676.1990.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Brunelli E., Risicato R., Cilluffo T., Jézéquel A. M., Orlandi F. Subcellular changes and apoptosis induced by ethanol in rat liver. J Hepatol. 1988 Apr;6(2):137–143. doi: 10.1016/s0168-8278(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Carthew P., Edwards R. E., Hill R. J., Evans J. G. Cytokeratin expression in cells of the rodent bile duct developing under normal and pathological conditions. Br J Exp Pathol. 1989 Dec;70(6):717–725. [PMC free article] [PubMed] [Google Scholar]
- Dempo K., Sasaki M., Kaku T., Satoh M., Oyamada M., Mori M. Immunohistochemical studies on alpha-fetoprotein- and albumin-containing cells in the liver during ontogenesis and early stage of 3'-Me-DAB hepatocarcinogenesis. Ann N Y Acad Sci. 1983;417:195–202. doi: 10.1111/j.1749-6632.1983.tb32863.x. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989 Mar 15;49(6):1541–1547. [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- GRISHAM J. W., HARTROFT W. S. Morphologic identification by electron microscopy of "oval" cells in experimental hepatic degeneration. Lab Invest. 1961 Mar-Apr;10:317–332. [PubMed] [Google Scholar]
- Gerber M. A., Thung S. N., Shen S., Stromeyer F. W., Ishak K. G. Phenotypic characterization of hepatic proliferation. Antigenic expression by proliferating epithelial cells in fetal liver, massive hepatic necrosis, and nodular transformation of the liver. Am J Pathol. 1983 Jan;110(1):70–74. [PMC free article] [PubMed] [Google Scholar]
- Germain L., Blouin M. J., Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, alpha-fetoprotein, albumin, and cell surface-exposed components. Cancer Res. 1988 Sep 1;48(17):4909–4918. [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Hayner N. T., Braun L., Yaswen P., Brooks M., Fausto N. Isozyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):332–338. [PubMed] [Google Scholar]
- Hixson D. C., Allison J. P. Monoclonal antibodies recognizing oval cells induced in the liver of rats by N-2-fluorenylacetamide or ethionine in a choline-deficient diet. Cancer Res. 1985 Aug;45(8):3750–3760. [PubMed] [Google Scholar]
- Mak K. M., Leo M. A., Lieber C. S. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984 Jul;87(1):188–200. [PubMed] [Google Scholar]
- POPPER H., SCHAFFNER F., HUTTERER F., PARONETTO F., BARKA T. Parenchymal fibrogenesis: the liver. Ann N Y Acad Sci. 1960 Jun 30;86:1075–1088. doi: 10.1111/j.1749-6632.1960.tb42863.x. [DOI] [PubMed] [Google Scholar]
- Plenat F., Braun L., Fausto N. Demonstration of glucose-6-phosphatase and peroxisomal catalase activity by ultrastructural cytochemistry in oval cells from livers of carcinogen-treated rats. Am J Pathol. 1988 Jan;130(1):91–102. [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Dwivedi R. S., Yeldandi A. V., Subbarao V., Tan X. D., Usman M. I., Thangada S., Nemali M. R., Kumar S., Scarpelli D. G. Role of periductal and ductular epithelial cells of the adult rat pancreas in pancreatic hepatocyte lineage. A change in the differentiation commitment. Am J Pathol. 1989 May;134(5):1069–1086. [PMC free article] [PubMed] [Google Scholar]
- Roskams T., van den Oord J. J., De Vos R., Desmet V. J. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990 Nov;137(5):1019–1025. [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S., Yachi A., Anzai T., Wada T. AFP-producing cells in hepatitis and in liver cirrhosis. Ann N Y Acad Sci. 1975 Aug 22;259:253–258. doi: 10.1111/j.1749-6632.1975.tb25421.x. [DOI] [PubMed] [Google Scholar]
- Scoazec J. Y., Moreau A., Feldmann G., Bernuau D. Cellular expression of alpha-fetoprotein gene and its relation to albumin gene expression during rat azo-dye hepatocarcinogenesis. Cancer Res. 1989 Apr 1;49(7):1790–1796. [PubMed] [Google Scholar]
- Sell S., Leffert H. L. An evaluation of cellular lineages in the pathogenesis of experimental hepatocellular carcinoma. Hepatology. 1982 Jan-Feb;2(1):77–86. doi: 10.1002/hep.1840020113. [DOI] [PubMed] [Google Scholar]
- Shah K. D., Gerber M. A. Development of intrahepatic bile ducts in humans. Immunohistochemical study using monoclonal cytokeratin antibodies. Arch Pathol Lab Med. 1989 Oct;113(10):1135–1138. [PubMed] [Google Scholar]
- Sirica A. E., Mathis G. A., Sano N., Elmore L. W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58(1):44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Uchida T., Peters R. L. The nature and origin of proliferated bile ductules in alcoholic liver disease. Am J Clin Pathol. 1983 Mar;79(3):326–333. doi: 10.1093/ajcp/79.3.326. [DOI] [PubMed] [Google Scholar]
- Vandersteenhoven A. M., Burchette J., Michalopoulos G. Characterization of ductular hepatocytes in end-stage cirrhosis. Arch Pathol Lab Med. 1990 Apr;114(4):403–406. [PubMed] [Google Scholar]
- Yaswen P., Hayner N. T., Fausto N. Isolation of oval cells by centrifugal elutriation and comparison with other cell types purified from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):324–331. [PubMed] [Google Scholar]
- Zajicek G., Ariel I., Arber N. The streaming liver. III. Littoral cells accompany the streaming hepatocyte. Liver. 1988 Aug;8(4):213–218. doi: 10.1111/j.1600-0676.1988.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Zajicek G., Oren R., Weinreb M., Jr The streaming liver. Liver. 1985 Dec;5(6):293–300. doi: 10.1111/j.1600-0676.1985.tb00252.x. [DOI] [PubMed] [Google Scholar]