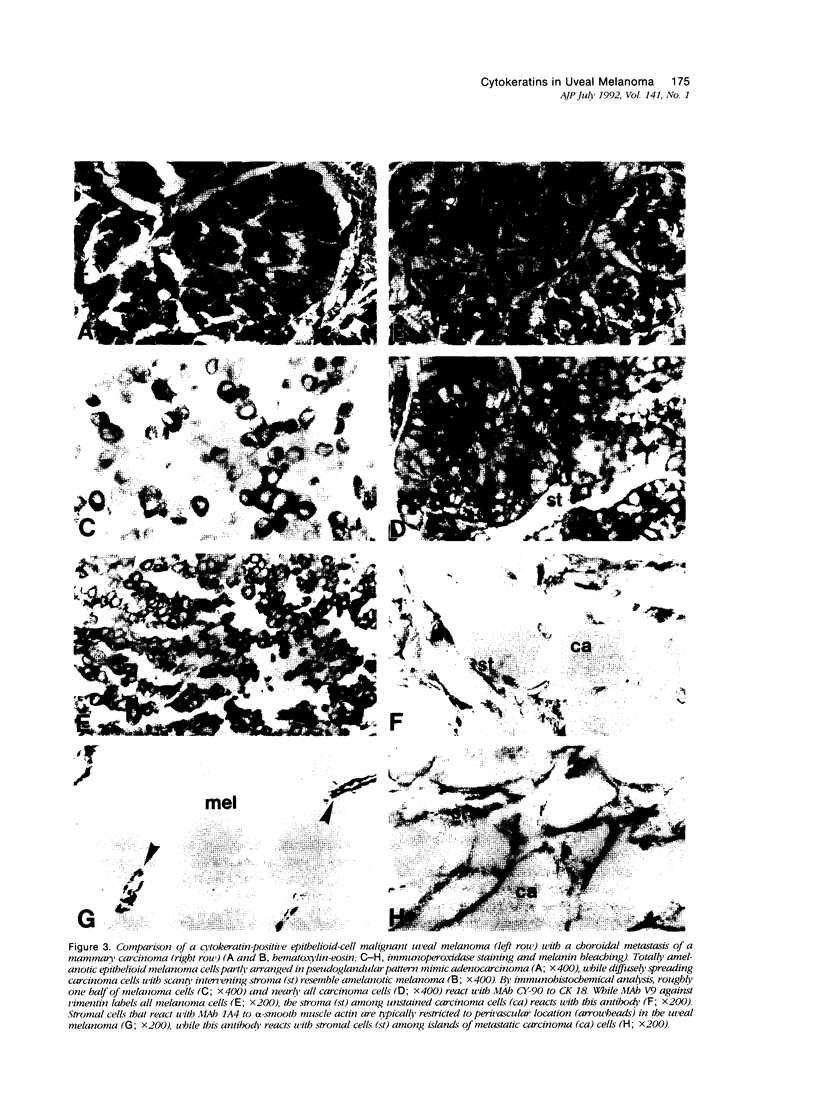
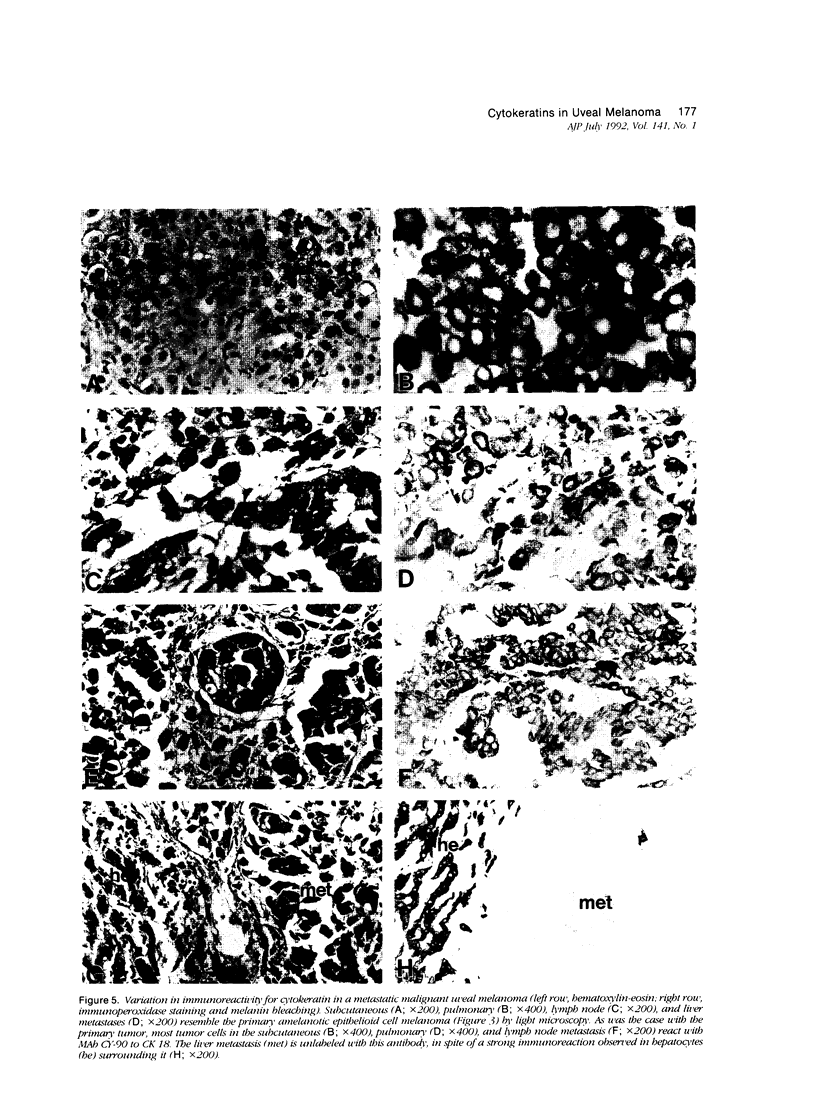
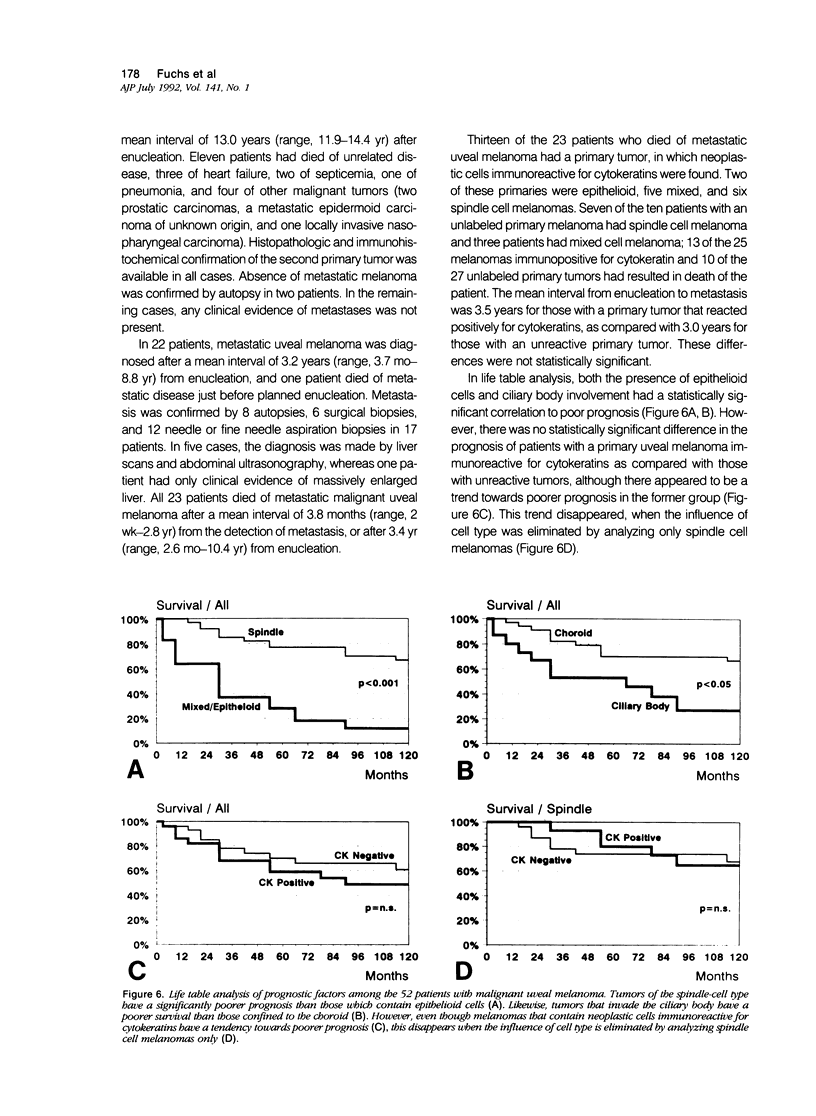
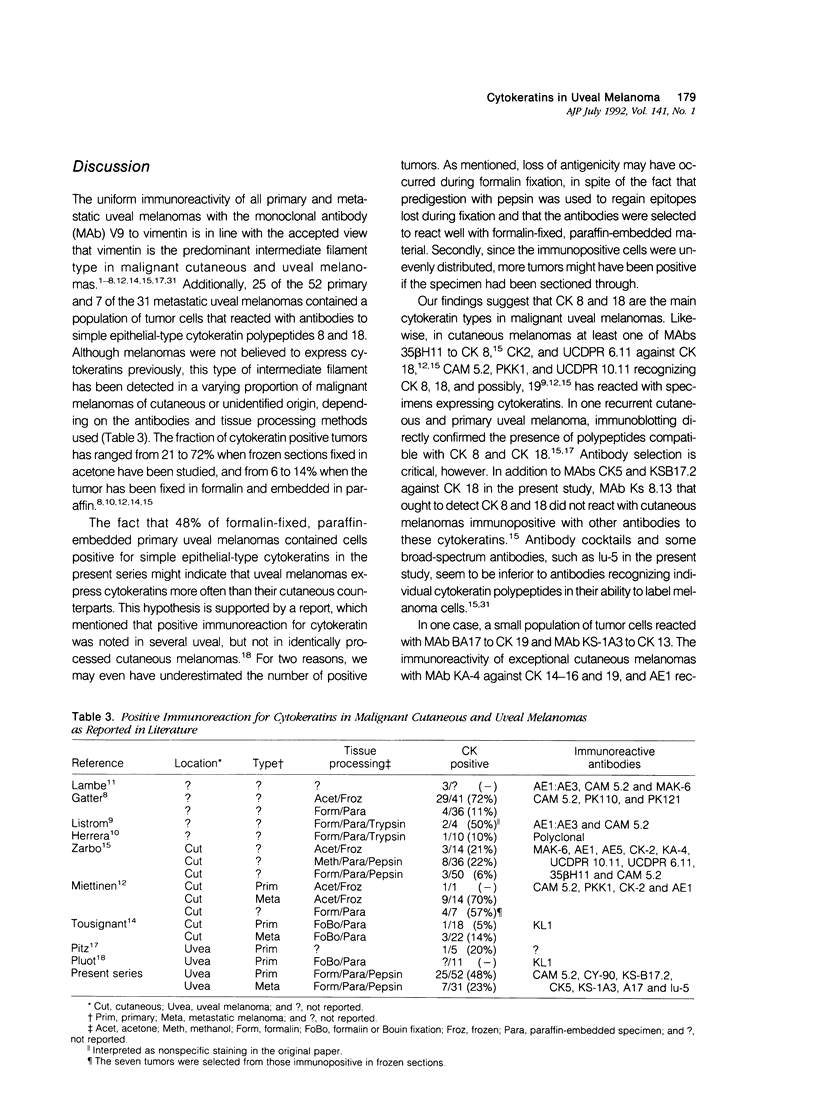
Abstract
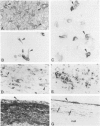
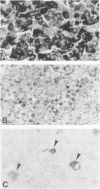
A group of 52 patients with malignant uveal melanoma treated by primary enucleation in 1977-1979 was studied to determine the frequency of immunoreactivity for cytokeratins (CK) in primary and metastatic melanoma, the CK types present, and the prognostic significance of CK expression. By immunohistochemistry, monoclonal antibody (MAb) V9 to vimentin reacted with all 52 formalin-fixed, paraffin-embedded primary tumors and all 31 metastases from 11 patients. MAb CAM 5.2 to CK 8 and 18 reacted with 20 and MAb CY-90 to CK 18 with 25 primary melanomas, whereas MAb KS-B17.2 and MAb CK5 to CK 18 labeled 8 and 6 tumors, respectively. Antibodies to CK 13 and CK 19 each labeled single cells in one specimen, and other CK types were not detected. In 6 primary melanomas, only a few tumor cells were immunopositive for CK 8 and 18, but in 17 cases up to one quarter, and in 2 tumors more than one quarter, of them were labeled. The positive cells were spindle, epithelioid, or intermediate in shape, and tended to be more frequent in mixed than in spindle cell melanomas. MAbs CAM 5.2 and CY-90 did not react with any of the 16 liver metastases, but labeled 7 of 15 other metastases. Metastases were somewhat more common when the primary tumor was immunoreactive for CK 8 and 18, apparently because CKs were more frequent in mixed cell melanomas. Although CK expression is of diagnostic significance and can denote low levels of epithelioid differentiation, it is not an independent prognostic factor in malignant uveal melanoma.
Full text
PDF



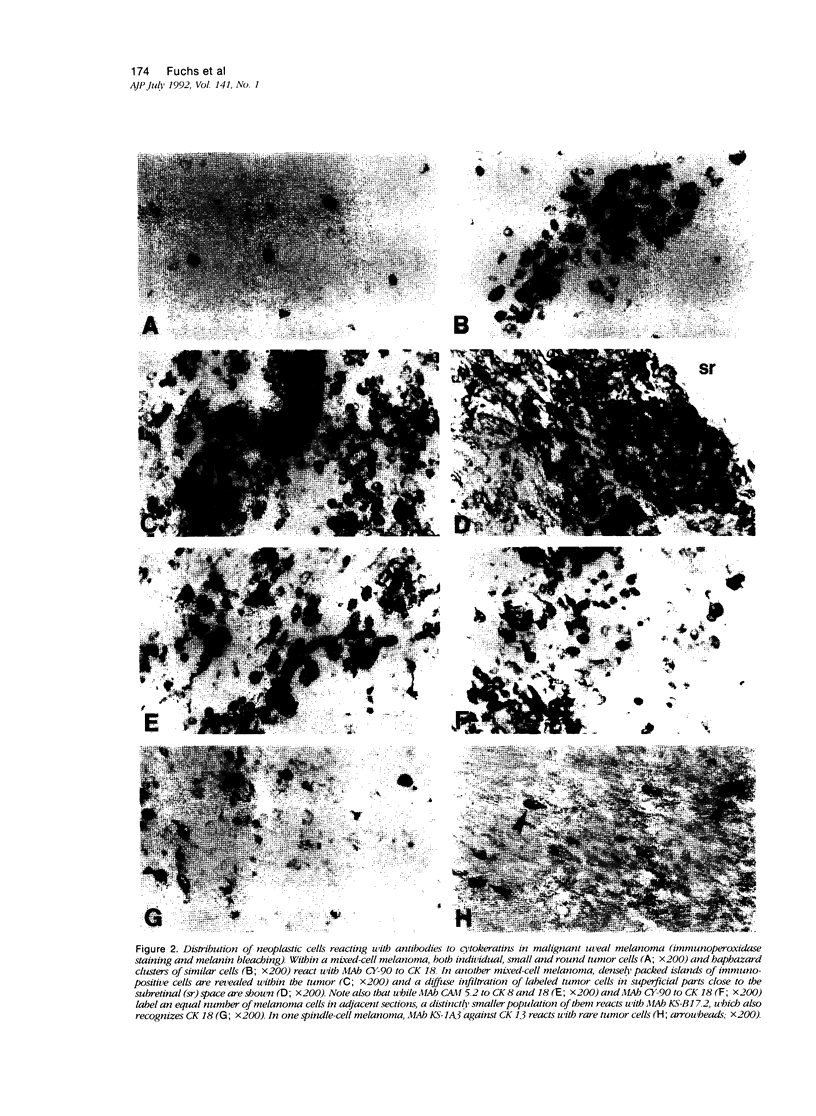



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azumi N., Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol. 1987 Sep;88(3):286–296. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- Bartek J., Durban E. M., Hallowes R. C., Taylor-Papadimitriou J. A subclass of luminal epithelial cells in the human mammary gland, defined by antibodies to cytokeratins. J Cell Sci. 1985 Apr;75:17–33. doi: 10.1242/jcs.75.1.17. [DOI] [PubMed] [Google Scholar]
- Caselitz J., Jänner M., Breitbart E., Weber K., Osborn M. Malignant melanomas contain only the vimentin type of intermediate filaments. Virchows Arch A Pathol Anat Histopathol. 1983;400(1):43–51. doi: 10.1007/BF00627007. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Moll R. Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation. 1987;36(2):145–163. doi: 10.1111/j.1432-0436.1987.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Winter S., von Overbeck J., Gudat F., Heitz P. U., Stähli C. Identification of the conserved, conformation-dependent cytokeratin epitope recognized by monoclonal antibody (lu-5). Virchows Arch A Pathol Anat Histopathol. 1987;411(2):137–147. doi: 10.1007/BF00712737. [DOI] [PubMed] [Google Scholar]
- Fuchs U., Kivelä T., Tarkkanen A. Cytoskeleton in normal and reactive human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1991 Dec;32(13):3178–3186. [PubMed] [Google Scholar]
- Gatter K. C., Ralfkiaer E., Skinner J., Brown D., Heryet A., Pulford K. A., Hou-Jensen K., Mason D. Y. An immunocytochemical study of malignant melanoma and its differential diagnosis from other malignant tumours. J Clin Pathol. 1985 Dec;38(12):1353–1357. doi: 10.1136/jcp.38.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Boyd H. C., Chang Y., Ferguson M., Reichler B., Tippens D. Smooth muscle cells can express cytokeratins of "simple" epithelium. Immunocytochemical and biochemical studies in vitro and in vivo. Am J Pathol. 1988 Aug;132(2):223–232. [PMC free article] [PubMed] [Google Scholar]
- Herrera G. A., Turbat-Herrera E. A., Lott R. L. S-100 protein expression by primary and metastatic adenocarcinomas. Am J Clin Pathol. 1988 Feb;89(2):168–176. doi: 10.1093/ajcp/89.2.168. [DOI] [PubMed] [Google Scholar]
- Huitfeldt H. S., Brandtzaeg P. Various keratin antibodies produce immunohistochemical staining of human myocardium and myometrium. Histochemistry. 1985;83(5):381–389. doi: 10.1007/BF00509196. [DOI] [PubMed] [Google Scholar]
- Huszar M., Halkin H., Herczeg E., Bubis J., Geiger B. Use of antibodies to intermediate filaments in the diagnosis of metastatic amelanotic malignant melanoma. Hum Pathol. 1983 Nov;14(11):1006–1008. doi: 10.1016/s0046-8177(83)80182-8. [DOI] [PubMed] [Google Scholar]
- Jahn L., Fouquet B., Rohe K., Franke W. W. Cytokeratins in certain endothelial and smooth muscle cells of two taxonomically distant vertebrate species, Xenopus laevis and man. Differentiation. 1987;36(3):234–254. doi: 10.1111/j.1432-0436.1987.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Jakobiec F. A., Silbert G. Are most iris "melanomas' really nevi? A clinicopathologic study of 189 lesions. Arch Ophthalmol. 1981 Dec;99(12):2117–2132. doi: 10.1001/archopht.1981.03930020993002. [DOI] [PubMed] [Google Scholar]
- Kan-Mitchell J., Rao N., Albert D. M., Van Eldik L. J., Taylor C. R. S100 immunophenotypes of uveal melanomas. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1492–1496. [PubMed] [Google Scholar]
- Leader M., Patel J., Makin C., Henry K. An analysis of the sensitivity and specificity of the cytokeratin marker CAM 5.2 for epithelial tumours. Results of a study of 203 sarcomas, 50 carcinomas and 28 malignant melanomas. Histopathology. 1986 Dec;10(12):1315–1324. doi: 10.1111/j.1365-2559.1986.tb02574.x. [DOI] [PubMed] [Google Scholar]
- Levy R., Czernobilsky B., Geiger B. Subtyping of epithelial cells of normal and metaplastic human uterine cervix, using polypeptide-specific cytokeratin antibodies. Differentiation. 1988 Dec;39(3):185–196. doi: 10.1111/j.1432-0436.1988.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Listrom M. B., Dalton L. W. Comparison of keratin monoclonal antibodies MAK-6, AE1:AE3, and CAM-5.2. Am J Clin Pathol. 1987 Sep;88(3):297–301. doi: 10.1093/ajcp/88.3.297. [DOI] [PubMed] [Google Scholar]
- Makin C. A., Bobrow L. G., Bodmer W. F. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984 Sep;37(9):975–983. doi: 10.1136/jcp.37.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Foster W. D., Zimmerman L. E. Prognostic factors in small malignant melanomas of choroid and ciliary body. Arch Ophthalmol. 1977 Jan;95(1):48–58. doi: 10.1001/archopht.1977.04450010050004. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Franssila K. Immunohistochemical spectrum of malignant melanoma. The common presence of keratins. Lab Invest. 1989 Dec;61(6):623–628. [PubMed] [Google Scholar]
- Nakhleh R. E., Wick M. R., Rocamora A., Swanson P. E., Dehner L. P. Morphologic diversity in malignant melanomas. Am J Clin Pathol. 1990 Jun;93(6):731–740. doi: 10.1093/ajcp/93.6.731. [DOI] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Pluot M., Joundi A., Grosshans E. Contribution de l'Ac monoclonal HMB45 au diagnostic histopathologique des mélanomes. Ann Dermatol Venereol. 1990;117(10):691–699. [PubMed] [Google Scholar]
- Ramaekers F. C., Puts J. J., Moesker O., Kant A., Vooijs G. P., Jap P. H. Intermediate filaments in malignant melanomas. Identification and use as marker in surgical pathology. J Clin Invest. 1983 Mar;71(3):635–643. doi: 10.1172/JCI110810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammas H. F., Blodi F. C. Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol. 1977 Jan;95(1):63–69. doi: 10.1001/archopht.1977.04450010065005. [DOI] [PubMed] [Google Scholar]
- Shoup S. A., Johnston W. W., Siegler H. F., Tello J. W., Schlom J., Bigner D. D., Bigner S. H. A panel of antibodies useful in the cytologic diagnosis of metastatic melanoma. Acta Cytol. 1990 May-Jun;34(3):385–392. [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate J., Williams H. K., Trejdosiewicz L. K., Hodges G. M. Primary culture of human oral epithelial cells. Growth requirements and expression of differentiated characteristics. Lab Invest. 1987 Feb;56(2):211–223. [PubMed] [Google Scholar]
- Toublanc M., Grossin M., Benrejeb N., Tousignant J., Crickx B., Belaich S., Bocquet L. Un cas de mélanome malin exprimant les marqueurs des cellules épithéliales en immunohistochimie sur coupes en paraffine. Ann Pathol. 1990;10(1):34–36. [PubMed] [Google Scholar]
- Tousignant J., Grossin M., Toublanc M., Dauge-Geffroy M. C., Bélaich S., Bocquet L. Les caractères immunohistochimiques des mélanomes malins. Etude de quarante cas et revue de la littérature. Arch Anat Cytol Pathol. 1990;38(1-2):5–10. [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Southgate J., Kemshead J. T., Hodges G. M. Phenotypic analysis of cultured melanoma cells. Expression of cytokeratin-type intermediate filaments by the M5 human melanoma cell line. Exp Cell Res. 1986 Jun;164(2):388–398. doi: 10.1016/0014-4827(86)90037-6. [DOI] [PubMed] [Google Scholar]
- Tölle H. G., Weber K., Osborn M. Microinjection of monoclonal antibodies specific for one intermediate filament protein in cells containing multiple keratins allow insight into the composition of particular 10 nm filaments. Eur J Cell Biol. 1985 Sep;38(2):234–244. [PubMed] [Google Scholar]
- Zarbo R. J., Gown A. M., Nagle R. B., Visscher D. W., Crissman J. D. Anomalous cytokeratin expression in malignant melanoma: one- and two-dimensional western blot analysis and immunohistochemical survey of 100 melanomas. Mod Pathol. 1990 Jul;3(4):494–501. [PubMed] [Google Scholar]
- von Overbeck J., Stähli C., Gudat F., Carmann H., Lautenschlager C., Dürmüller U., Takacs B., Miggiano V., Staehelin T., Heitz P. U. Immunohistochemical characterization of an anti-epithelial monoclonal antibody (mAB lu-5). Virchows Arch A Pathol Anat Histopathol. 1985;407(1):1–12. doi: 10.1007/BF00701324. [DOI] [PubMed] [Google Scholar]