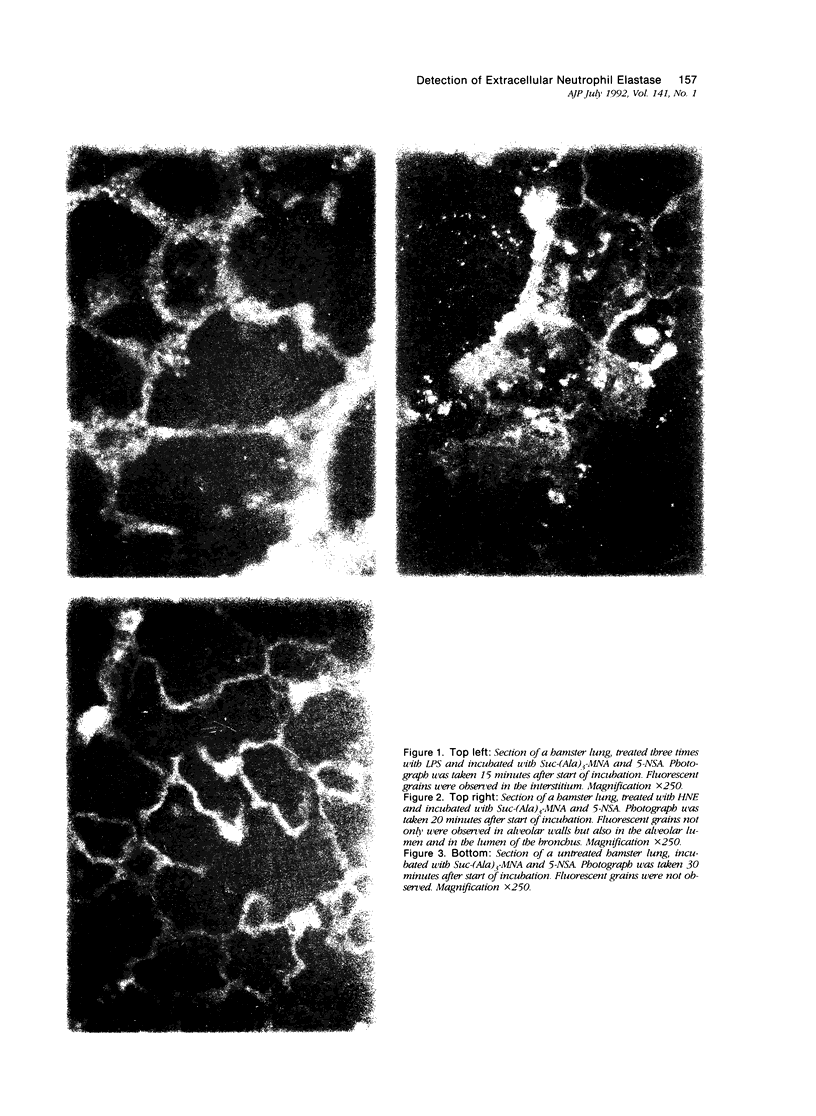
Abstract
Repeated intratracheal instillations of E. coli lipopolysaccharide (LPS) in hamster lungs cause an influx of polymorphonuclear leukocytes (PMNs) into the alveolar walls, with concomitant development of severe emphysema. It has been suggested that elastase, released by these PMNs, is involved in the development of emphysema. This study demonstrates the release of elastase from recruited PMNs in cryostat sections of hamster lungs, after being treated once, twice, or thrice with LPS, intratracheally. Elastase activity was visualized using two elastase-specific synthetic substrates, to which a methoxynaphthylamine (MNA) group had been bound covalently. Liberated MNA, when made insoluble by coupling with 5-nitrosalicylaldehyde, fluoresces strongly. The authors observed that the interval between start of incubation and appearance of fluorescence and the intensity of fluorescence correlated with the number of LPS administrations. Fluorescence was observed to be located in or in close vicinity to alveolar walls. No fluorescence was observed in sections of untreated hamsters. Liberation of MNA from synthetic substrates was delayed strongly by the addition of a recombinant secretory leukocyte proteinase inhibitor or a substituted cephalosporin neutrophil elastase inhibitor. The authors conclude that LPS-mediated PMN influx into the lung is accompanied by release of elastase from these cells and speculate that this PMN-elastase is involved in the development of LPS-mediated emphysema.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Boudier C., Bieth J. G. Estimation and characterization of hamster leukocyte elastase. Biochem Med. 1982 Aug;28(1):41–50. doi: 10.1016/0006-2944(82)90053-9. [DOI] [PubMed] [Google Scholar]
- Boudier C., Bieth J. G. Mucus proteinase inhibitor: a fast-acting inhibitor of leucocyte elastase. Biochim Biophys Acta. 1989 Mar 16;995(1):36–41. doi: 10.1016/0167-4838(89)90230-6. [DOI] [PubMed] [Google Scholar]
- Brogden K. A., Cutlip R. C., Lehmkuhl H. D. Response of sheep after localized deposition of lipopolysaccharide in the lung. Exp Lung Res. 1984;7(2):123–132. doi: 10.3109/01902148409069673. [DOI] [PubMed] [Google Scholar]
- Burrell R., Lantz R. C., Hinton D. E. Mediators of pulmonary injury induced by inhalation of bacterial endotoxin. Am Rev Respir Dis. 1988 Jan;137(1):100–105. doi: 10.1164/ajrccm/137.1.100. [DOI] [PubMed] [Google Scholar]
- Davies P., Ashe B. M., Bonney R. J., Dorn C., Finke P., Fletcher D., Hanlon W. A., Humes J. L., Maycock A., Mumford R. A. The discovery and biologic properties of cephalosporin-based inhibitors of PMN elastase. Ann N Y Acad Sci. 1991;624:219–229. doi: 10.1111/j.1749-6632.1991.tb17021.x. [DOI] [PubMed] [Google Scholar]
- Del Mar E. G., Largman C., Brodrick J. W., Fassett M., Geokas M. C. Substrate specificity of human pancreatic elastase 2. Biochemistry. 1980 Feb 5;19(3):468–472. doi: 10.1021/bi00544a011. [DOI] [PubMed] [Google Scholar]
- Dolbeare F. A., Smith R. E. Flow cytometric measurement of peptidases with use of 5-nitrosalicylaldehyde and 4-methoxy-beta-naphthylamine derivatives. Clin Chem. 1977 Aug;23(8):1485–1491. [PubMed] [Google Scholar]
- Dolbeare F., Vanderlaan M. A fluorescent assay of proteinases in cultured mammalian cells. J Histochem Cytochem. 1979 Nov;27(11):1493–1495. doi: 10.1177/27.11.512330. [DOI] [PubMed] [Google Scholar]
- Feinstein G., Janoff A. A rapid method of purification of human granulocyte cationic neutral proteases: purification and further characterization of human granulocyte elastase. Biochim Biophys Acta. 1975 Oct 22;403(2):493–505. doi: 10.1016/0005-2744(75)90077-7. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Henricks P. A., Slootweg P. J., Nijkamp F. P. Endotoxin-induced inflammation and injury of the guinea pig respiratory airways cause bronchial hyporeactivity. Am Rev Respir Dis. 1988 Jun;137(6):1441–1448. doi: 10.1164/ajrccm/137.6.1441. [DOI] [PubMed] [Google Scholar]
- Gauthier F., Fryksmark U., Ohlsson K., Bieth J. G. Kinetics of the inhibition of leukocyte elastase by the bronchial inhibitor. Biochim Biophys Acta. 1982 Jan 18;700(2):178–183. doi: 10.1016/0167-4838(82)90095-4. [DOI] [PubMed] [Google Scholar]
- Haslett C., Worthen G. S., Giclas P. C., Morrison D. C., Henson J. E., Henson P. M. The pulmonary vascular sequestration of neutrophils in endotoxemia is initiated by an effect of endotoxin on the neutrophil in the rabbit. Am Rev Respir Dis. 1987 Jul;136(1):9–18. doi: 10.1164/ajrccm/136.1.9. [DOI] [PubMed] [Google Scholar]
- Hudson A. R., Kilburn K. H., Halprin G. M., McKenzie W. N. Granulocyte recruitment to airways exposed to endotoxin aerosols. Am Rev Respir Dis. 1977 Jan;115(1):89–95. doi: 10.1164/arrd.1977.115.1.89. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., van Twisk C., Klasen E. C., Dijkman J. H. Interactions among stimulated human polymorphonuclear leucocytes, released elastase and bronchial antileucoprotease. Clin Sci (Lond) 1988 Jul;75(1):53–62. doi: 10.1042/cs0750053. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., van der Valk P., van der Sandt M. M., Lindeman J., Meijer C. J. Elastase as a marker for neutrophilic myeloid cells. J Histochem Cytochem. 1984 Apr;32(4):389–394. doi: 10.1177/32.4.6561228. [DOI] [PubMed] [Google Scholar]
- Milton D. K., Godleski J. J., Feldman H. A., Greaves I. A. Toxicity of intratracheally instilled cotton dust, cellulose, and endotoxin. Am Rev Respir Dis. 1990 Jul;142(1):184–192. doi: 10.1164/ajrccm/142.1.184. [DOI] [PubMed] [Google Scholar]
- Morrison H. M. The proteinase-antiproteinase theory of emphysema: time for a reappraisal? Clin Sci (Lond) 1987 Feb;72(2):151–158. doi: 10.1042/cs0720151. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Tegner H. Inhibition of elastase from granulocytes by the low molecular weight bronchial protease inhibitor. Scand J Clin Lab Invest. 1976 Sep;36(5):437–445. doi: 10.3109/00365517609054461. [DOI] [PubMed] [Google Scholar]
- Rylander R., Marchat B. Modulation of acute endotoxin pulmonary inflammation by a corticosteroid. J Clin Lab Immunol. 1988 Oct;27(2):83–86. [PubMed] [Google Scholar]
- Smith C. E., Johnson D. A. Human bronchial leucocyte proteinase inhibitor. Rapid isolation and kinetic analysis with human leucocyte proteinases. Biochem J. 1985 Jan 15;225(2):463–472. doi: 10.1042/bj2250463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snella M. C., Rylander R. Lung cell reactions after inhalation of bacterial lipopolysaccharides. Eur J Respir Dis. 1982 Nov;63(6):550–557. [PubMed] [Google Scholar]
- Snider G. L. Experimental studies on emphysema and chronic bronchial injury. Eur J Respir Dis Suppl. 1986;146:17–35. [PubMed] [Google Scholar]
- Starcher B., Williams I. A method for intratracheal instillation of endotoxin into the lungs of mice. Lab Anim. 1989 Jul;23(3):234–240. doi: 10.1258/002367789780810536. [DOI] [PubMed] [Google Scholar]
- Starcher B., Williams I. The beige mouse: role of neutrophil elastase in the development of pulmonary emphysema. Exp Lung Res. 1989 Sep;15(5):785–800. doi: 10.3109/01902148909062861. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Adles C., Hibbs J. B., Jr Cloned macrophages can be induced to kill tumor cells. J Reticuloendothel Soc. 1978 Aug;24(2):107–113. [PubMed] [Google Scholar]
- Stolk J., Rudolphus A., Kramps J. A. Lipopolysaccharide-induced alveolar wall destruction in the hamster is inhibited by intratracheal treatment with r-secretory leukocyte protease inhibitor. Ann N Y Acad Sci. 1991;624:350–352. doi: 10.1111/j.1749-6632.1991.tb17045.x. [DOI] [PubMed] [Google Scholar]
- Twumasi D. Y., Liener I. E. Proteases from purulent sputum. Purification and properties of the elastase and chymotrypsin-like enzymes. J Biol Chem. 1977 Mar 25;252(6):1917–1926. [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M., Everts V., Beertsen W. Localization of cathepsin B activity in fibroblasts and chondrocytes by continuous monitoring of the formation of a final fluorescent reaction product using 5-nitrosalicylaldehyde. Histochem J. 1987 Sep;19(9):483–487. doi: 10.1007/BF01675418. [DOI] [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M., Smith R. E. Localization and cytophotometric analysis of cathepsin B activity in unfixed and undecalcified cryostat sections of whole rat knee joints. J Histochem Cytochem. 1989 May;37(5):617–624. doi: 10.1177/37.5.2703699. [DOI] [PubMed] [Google Scholar]
- Venaille T., Snella M. C., Holt P. G., Rylander R. Cell recruitment into lung wall and airways of conventional and pathogen-free guinea pigs after inhalation of endotoxin. Am Rev Respir Dis. 1989 Jun;139(6):1356–1360. doi: 10.1164/ajrccm/139.6.1356. [DOI] [PubMed] [Google Scholar]
- Wewers M. Pathogenesis of emphysema. Assessment of basic science concepts through clinical investigation. Chest. 1989 Jan;95(1):190–195. doi: 10.1378/chest.95.1.190. [DOI] [PubMed] [Google Scholar]