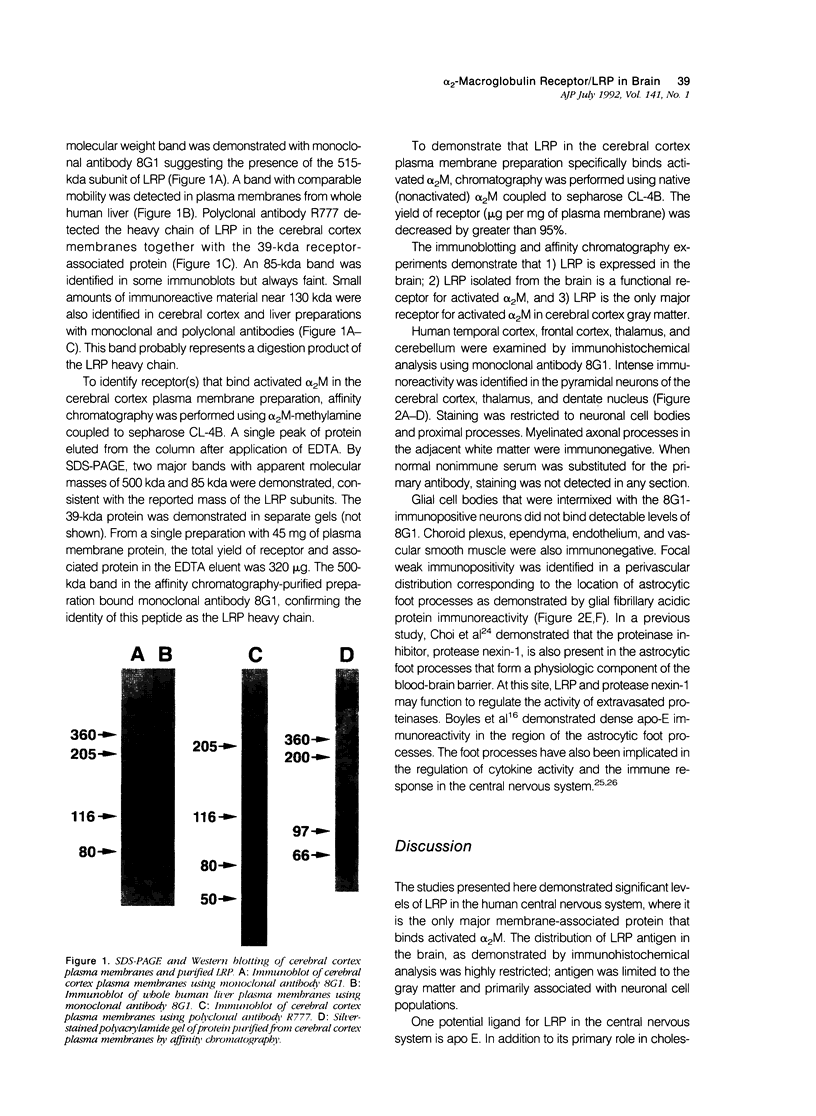
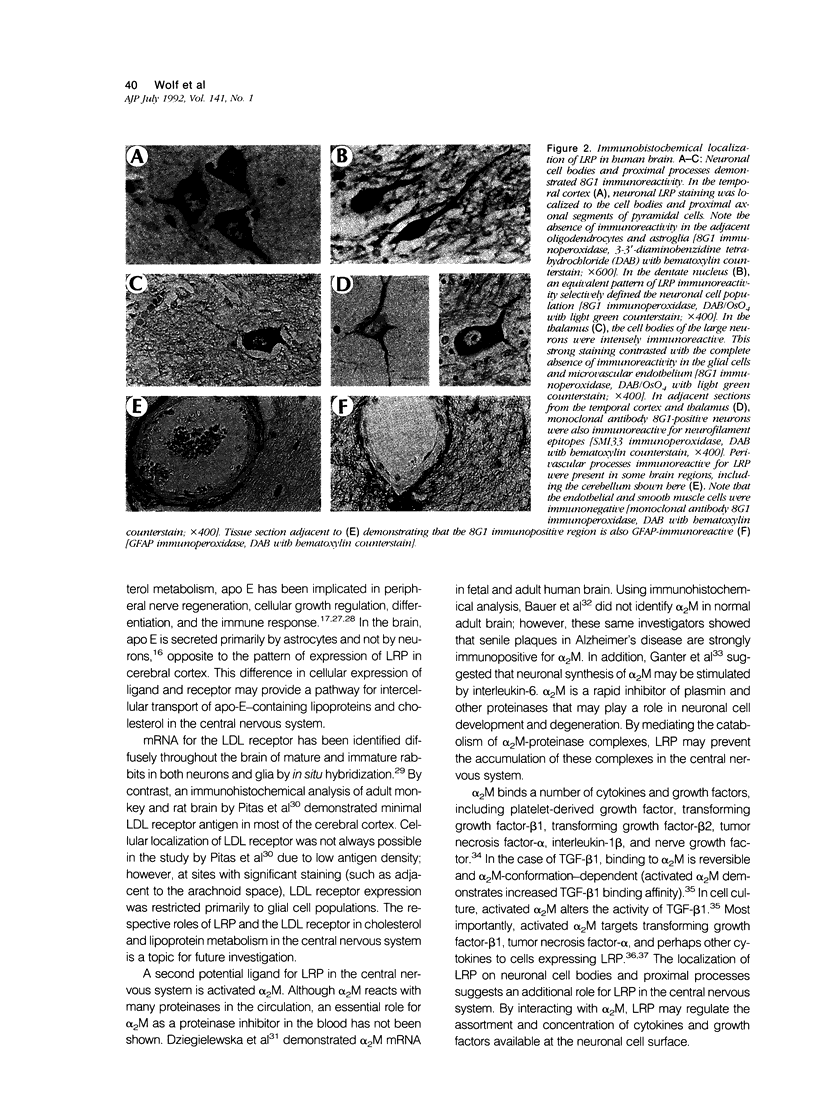
Abstract
Proteinase inhibitors have been implicated in brain development and in degenerative processes such as Alzheimer's disease. Low-density lipoprotein receptor-related protein (LRP) is a multifunctional cell-surface receptor that binds activated forms of the proteinase inhibitor, alpha 2-macroglobulin (alpha 2M) and apolipoprotein E. Solubilized plasma membranes of human cerebral cortical gray matter were subjected to affinity chromatography on alpha 2M-methylamine-sepharose. A single receptor was purified; this protein was LRP as determined by molecular mass, peptide structure, and immunoreactivity with monoclonal and polyclonal antibodies. In adult human brain, LRP immunoreactivity was abundant on neuronal cell bodies and proximal processes. Other cells within the neuropil, including glia and microvascular cells (endothelium and pericytes), were immunonegative. Weak LRP immunoreactivity was identified in a perivascular pattern corresponding to the location of astrocytic foot processes. The distribution of LRP in the central nervous system is consistent with the potential function of this receptor in the regulation of proteinase activity, cytokine activity, and cholesterol metabolism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcom J. D., Tiller S. E., Dickerson K., Cravens J. L., Argraves W. S., Strickland D. K. The human alpha 2-macroglobulin receptor: identification of a 420-kD cell surface glycoprotein specific for the activated conformation of alpha 2-macroglobulin. J Cell Biol. 1990 Apr;110(4):1041–1048. doi: 10.1083/jcb.110.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J., Strauss S., Schreiter-Gasser U., Ganter U., Schlegel P., Witt I., Yolk B., Berger M. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer's disease cortices. FEBS Lett. 1991 Jul 8;285(1):111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- Beisiegel U., Weber W., Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8342–8346. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles J. K., Pitas R. E., Wilson E., Mahley R. W., Taylor J. M. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985 Oct;76(4):1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B. H., Suzuki M., Kim T., Wagner S. L., Cunningham D. D. Protease nexin-1. Localization in the human brain suggests a protective role against extravasated serine proteases. Am J Pathol. 1990 Oct;137(4):741–747. [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Saunders N. R., Schejter E. J., Zakut H., Zevin-Sonkin D., Zisling R., Soreq H. Synthesis of plasma proteins in fetal, adult, and neoplastic human brain tissue. Dev Biol. 1986 May;115(1):93–104. doi: 10.1016/0012-1606(86)90231-9. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- Ganter U., Strauss S., Jonas U., Weidemann A., Beyreuther K., Volk B., Berger M., Bauer J. Alpha 2-macroglobulin synthesis in interleukin-6-stimulated human neuronal (SH-SY5Y neuroblastoma) cells. Potential significance for the processing of Alzheimer beta-amyloid precursor protein. FEBS Lett. 1991 Apr 22;282(1):127–131. doi: 10.1016/0014-5793(91)80460-k. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W. Endothelial cell-astrocyte interactions. A cellular model of the blood-brain barrier. Ann N Y Acad Sci. 1988;529:31–39. doi: 10.1111/j.1749-6632.1988.tb51417.x. [DOI] [PubMed] [Google Scholar]
- Gonias S. L. Alpha 2-macroglobulin: a protein at the interface of fibrinolysis and cellular growth regulation. Exp Hematol. 1992 Mar;20(3):302–311. [PubMed] [Google Scholar]
- Gonias S. L., Figler N. L. Alpha 2-macroglobulin is the primary inhibitor of miniplasmin in vitro and in vivo in the mouse. Comparison with alpha 2-antiplasmin in simultaneous reaction experiments. Biochem J. 1988 Oct 15;255(2):725–730. [PMC free article] [PubMed] [Google Scholar]
- Hall S. W., LaMarre J., Marshall L. B., Hayes M. A., Gonias S. L. Binding of transforming growth factor-beta 1 to methylamine-modified alpha 2-macroglobulin and to binary and ternary alpha 2-macroglobulin-proteinase complexes. Biochem J. 1992 Jan 15;281(Pt 2):569–575. doi: 10.1042/bj2810569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C., Guilbert L. J., Fedoroff S. Production of colony-stimulating factor-1 (CSF-1) by mouse astroglia in vitro. J Neurosci Res. 1990 Nov;27(3):314–323. doi: 10.1002/jnr.490270310. [DOI] [PubMed] [Google Scholar]
- Herz J., Goldstein J. L., Strickland D. K., Ho Y. K., Brown M. S. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991 Nov 5;266(31):21232–21238. [PubMed] [Google Scholar]
- Herz J., Kowal R. C., Goldstein J. L., Brown M. S. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990 Jun;9(6):1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hubbard C. M., Redpath G. T., Macdonald T. L., VandenBerg S. R. Modulatory effects of aluminum, calcium, lithium, magnesium, and zinc ions on [3H]MK-801 binding in human cerebral cortex. Brain Res. 1989 May 1;486(1):170–174. doi: 10.1016/0006-8993(89)91290-0. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Shooter E. M., Pitas R. E., Mahley R. W. Lipoprotein uptake by neuronal growth cones in vitro. Science. 1987 May 22;236(4804):959–962. doi: 10.1126/science.3576212. [DOI] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Jensen P. H., Moestrup S. K., Gliemann J. Purification of the human placental alpha 2-macroglobulin receptor. FEBS Lett. 1989 Sep 25;255(2):275–280. doi: 10.1016/0014-5793(89)81105-6. [DOI] [PubMed] [Google Scholar]
- Kristensen T., Moestrup S. K., Gliemann J., Bendtsen L., Sand O., Sottrup-Jensen L. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the alpha 2-macroglobulin receptor. FEBS Lett. 1990 Dec 10;276(1-2):151–155. doi: 10.1016/0014-5793(90)80530-v. [DOI] [PubMed] [Google Scholar]
- LaMarre J., Hayes M. A., Wollenberg G. K., Hussaini I., Hall S. W., Gonias S. L. An alpha 2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor-beta 1 in mice. J Clin Invest. 1991 Jan;87(1):39–44. doi: 10.1172/JCI114998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Analysis of ligand recognition by the purified alpha 2-macroglobulin receptor (low density lipoprotein receptor-related protein). Evidence that high affinity of alpha 2-macroglobulin-proteinase complex is achieved by binding to adjacent receptors. J Biol Chem. 1991 Jul 25;266(21):14011–14017. [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Purification of the rat hepatic alpha 2-macroglobulin receptor as an approximately 440-kDa single chain protein. J Biol Chem. 1989 Sep 15;264(26):15574–15577. [PubMed] [Google Scholar]
- Pitas R. E., Boyles J. K., Lee S. H., Hui D., Weisgraber K. H. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987 Oct 15;262(29):14352–14360. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Gliemann J., Van Leuven F. Domain structure of human alpha 2-macroglobulin. Characterization of a receptor-binding domain obtained by digestion with papain. FEBS Lett. 1986 Sep 1;205(1):20–24. doi: 10.1016/0014-5793(86)80857-2. [DOI] [PubMed] [Google Scholar]
- Strickland D. K., Ashcom J. D., Williams S., Battey F., Behre E., McTigue K., Battey J. F., Argraves W. S. Primary structure of alpha 2-macroglobulin receptor-associated protein. Human homologue of a Heymann nephritis antigen. J Biol Chem. 1991 Jul 15;266(20):13364–13369. [PubMed] [Google Scholar]
- Strickland D. K., Ashcom J. D., Williams S., Burgess W. H., Migliorini M., Argraves W. S. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990 Oct 15;265(29):17401–17404. [PubMed] [Google Scholar]
- Swanson L. W., Simmons D. M., Hofmann S. L., Goldstein J. L., Brown M. S. Localization of mRNA for low density lipoprotein receptor and a cholesterol synthetic enzyme in rabbit nervous system by in situ hybridization. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9821–9825. doi: 10.1073/pnas.85.24.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leuven F., Marynen P., Sottrup-Jensen L., Cassiman J. J., Van den Berghe H. The receptor-binding domain of human alpha 2-macroglobulin. Isolation after limited proteolysis with a bacterial proteinase. J Biol Chem. 1986 Aug 25;261(24):11369–11373. [PubMed] [Google Scholar]
- Wollenberg G. K., LaMarre J., Rosendal S., Gonias S. L., Hayes M. A. Binding of tumor necrosis factor alpha to activated forms of human plasma alpha 2 macroglobulin. Am J Pathol. 1991 Feb;138(2):265–272. [PMC free article] [PubMed] [Google Scholar]