Abstract
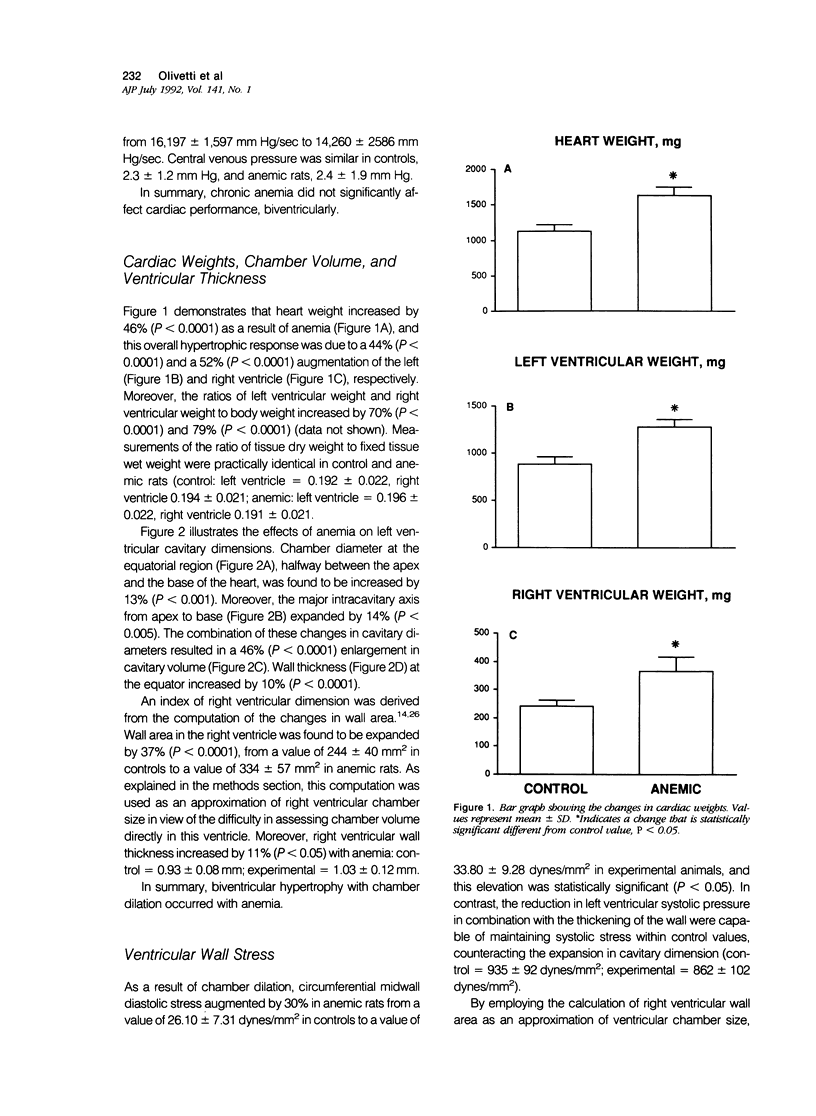
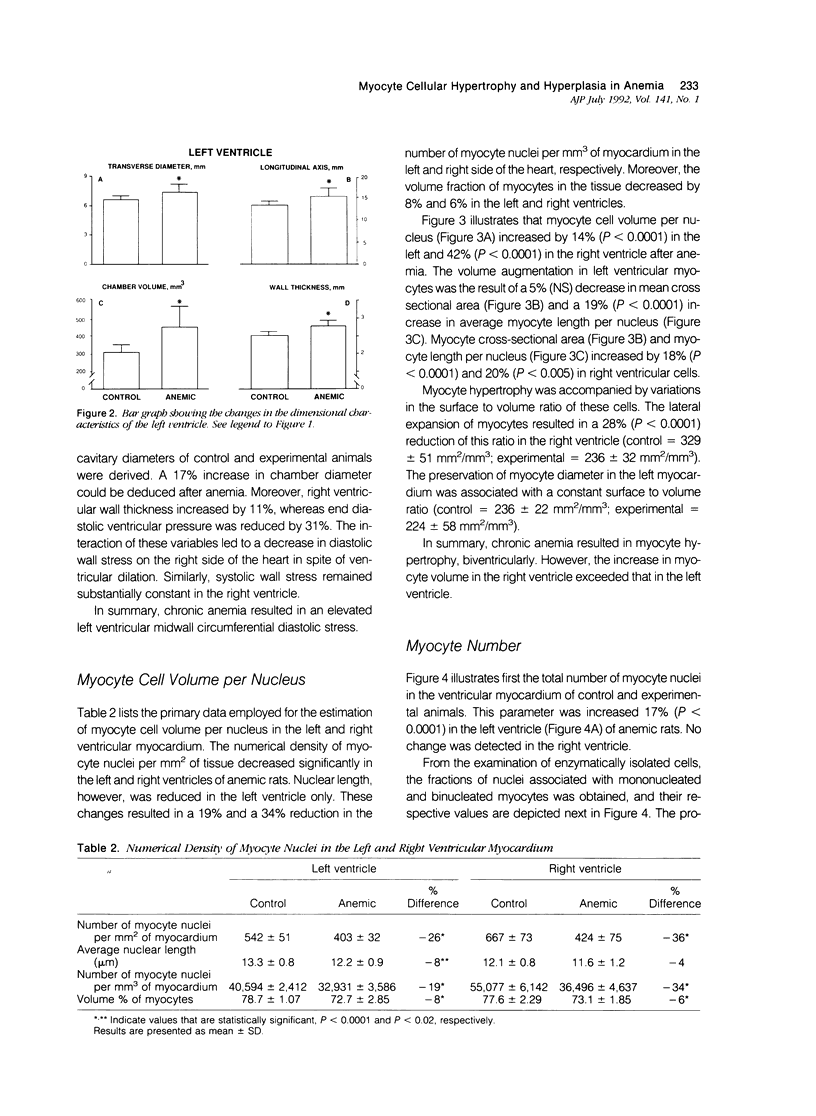
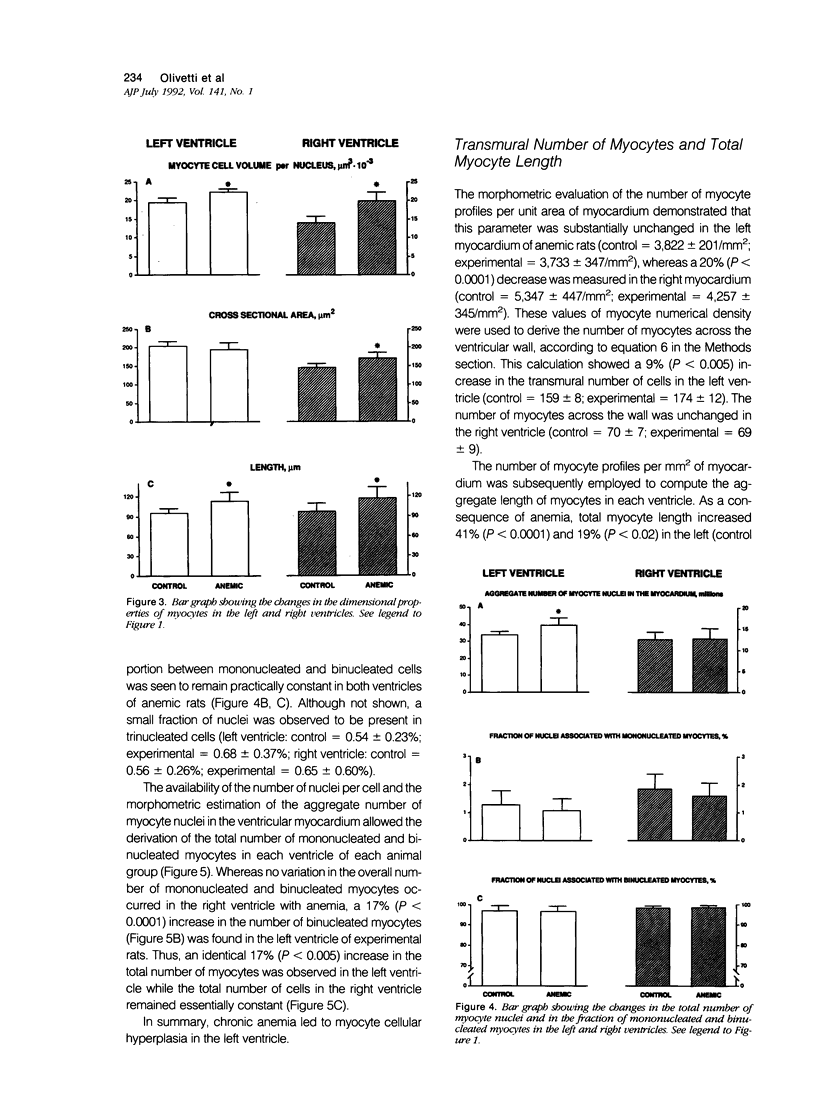
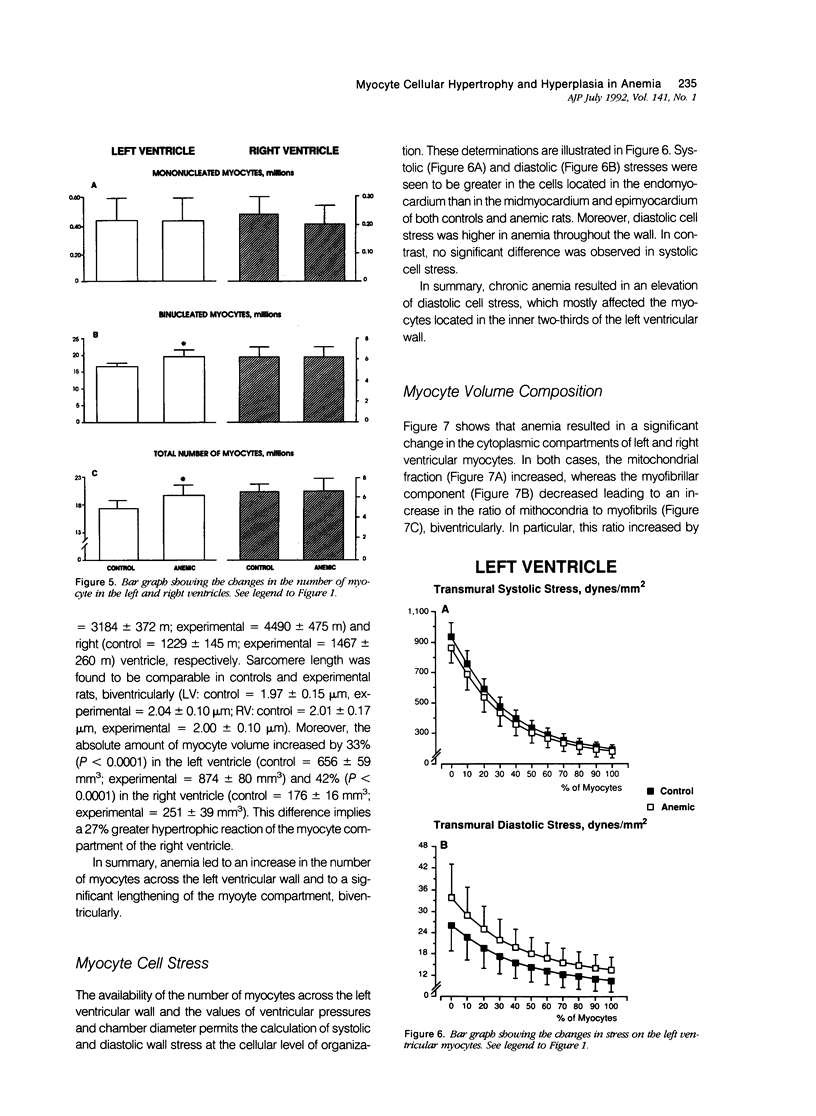
To determine the effects of chronic anemia on the functional and structural characteristics of the heart, 1-month-old male rats were fed a diet deficient in iron and copper, which led to a hemoglobin concentration of 4.63 g/dl, for 8 weeks. At sacrifice, under fentanyl citrate and droperidol anesthesia, systolic, diastolic, and mean arterial blood pressures were decreased, whereas differential pressure was increased. Left ventricular systolic pressure and the ventricular rate of pressure rise (mmHg/s) were reduced by 9% and 14%, respectively. Moreover, developed peak systolic ventricular pressure and maximal dP/dt diminished 14% and 12%. After perfusion fixation of the coronary vasculature and the myocardium, at a left ventricular intracavitary pressure equal to the in vivo measured end diastolic pressure, a 10% thickening of the left ventricular wall was measured in association with a 13% increase in the equatorial cavitary diameter and a 44% augmentation in ventricular mass. The 52% hypertrophy of the right ventricle was characterized by an 11% thicker wall and a 37% larger ventricular area. The 33% expansion in the aggregate myocyte volume of the left ventricle was found to be due to a 14% myocyte cellular hypertrophy and a 17% myocyte cellular hyperplasia. These cellular parameters were calculated from the estimation of the number of myocyte nuclei per unit volume of myocardium in situ and the evaluation of the distribution of nuclei per cell in enzymatically dissociated myocytes. Myocyte cellular hyperplasia provoked a 9% increase in the absolute number of cells across the left ventricular wall. In contrast, myocyte cellular hypertrophy (42%) was responsible for the increase in myocyte volume of the right ventricle. The proliferative response of left ventricular myocytes was not capable of restoring diastolic cell stress, which was enhanced by the changes in ventricular anatomy with anemia. In conclusion, chronic anemia induced an unbalanced load on the left ventricle, which evoked a hyperplastic reaction of preexisting myocytes, in an attempt to normalize diastolic wall and myocyte stress.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anversa P., Beghi C., Kikkawa Y., Olivetti G. Myocardial response to infarction in the rat. Morphometric measurement of infarct size and myocyte cellular hypertrophy. Am J Pathol. 1985 Mar;118(3):484–492. [PMC free article] [PubMed] [Google Scholar]
- Anversa P., Beghi C., Levicky V., McDonald S. L., Kikkawa Y. Morphometry of right ventricular hypertrophy induced by strenuous exercise in rat. Am J Physiol. 1982 Dec;243(6):H856–H861. doi: 10.1152/ajpheart.1982.243.6.H856. [DOI] [PubMed] [Google Scholar]
- Anversa P., Beghi C., Levicky V., McDonald S. L., Kikkawa Y., Olivetti G. Effects of strenuous exercise on the quantitative morphology of left ventricular myocardium in the rat. J Mol Cell Cardiol. 1985 Jun;17(6):587–595. doi: 10.1016/s0022-2828(85)80027-4. [DOI] [PubMed] [Google Scholar]
- Anversa P., Levicky V., Beghi C., McDonald S. L., Kikkawa Y. Morphometry of exercise-induced right ventricular hypertrophy in the rat. Circ Res. 1983 Jan;52(1):57–64. doi: 10.1161/01.res.52.1.57. [DOI] [PubMed] [Google Scholar]
- Anversa P., Palackal T., Sonnenblick E. H., Olivetti G., Capasso J. M. Hypertensive cardiomyopathy. Myocyte nuclei hyperplasia in the mammalian rat heart. J Clin Invest. 1990 Apr;85(4):994–997. doi: 10.1172/JCI114576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P., Palackal T., Sonnenblick E. H., Olivetti G., Meggs L. G., Capasso J. M. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990 Oct;67(4):871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- Anversa P., Ricci R., Olivetti G. Coronary capillaries during normal and pathological growth. Can J Cardiol. 1986 Mar-Apr;2(2):104–113. [PubMed] [Google Scholar]
- Anversa P., Ricci R., Olivetti G. Quantitative structural analysis of the myocardium during physiologic growth and induced cardiac hypertrophy: a review. J Am Coll Cardiol. 1986 May;7(5):1140–1149. doi: 10.1016/s0735-1097(86)80236-4. [DOI] [PubMed] [Google Scholar]
- Anversa P., Sonnenblick E. H. Ischemic cardiomyopathy: pathophysiologic mechanisms. Prog Cardiovasc Dis. 1990 Jul-Aug;33(1):49–70. doi: 10.1016/0033-0620(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Bartosová D., Chvapil M., Korecký B., Poupa O., Rakusan K., Turek Z., Vízek M. The growth of the muscular and collagenous parts of the rat heart in various forms of cardiomegaly. J Physiol. 1969 Feb;200(2):285–295. doi: 10.1113/jphysiol.1969.sp008693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M. L. Warner heart oration (1978). Circulatory adaptations in chronic severe anaemia. Indian Heart J. 1979 May-Jun;31(3):132–137. [PubMed] [Google Scholar]
- Capasso J. M., Palackal T., Olivetti G., Anversa P. Left ventricular failure induced by long-term hypertension in rats. Circ Res. 1990 May;66(5):1400–1412. doi: 10.1161/01.res.66.5.1400. [DOI] [PubMed] [Google Scholar]
- Capasso J. M., Palackal T., Olivetti G., Anversa P. Severe myocardial dysfunction induced by ventricular remodeling in aging rat hearts. Am J Physiol. 1990 Oct;259(4 Pt 2):H1086–H1096. doi: 10.1152/ajpheart.1990.259.4.H1086. [DOI] [PubMed] [Google Scholar]
- Claycomb W. C. Biochemical aspects of cardiac muscle differentiation. Deoxyribonucleic acid synthesis and nuclear and cytoplasmic deoxyribonucleic acid polymerase activity. J Biol Chem. 1975 May 10;250(9):3229–3235. [PubMed] [Google Scholar]
- Claycomb W. C., Moses R. L. Growth factors and TPA stimulate DNA synthesis and alter the morphology of cultured terminally differentiated adult rat cardiac muscle cells. Dev Biol. 1988 Jun;127(2):257–265. doi: 10.1016/0012-1606(88)90313-2. [DOI] [PubMed] [Google Scholar]
- Datta B. N., Silver M. D. Cardiomegaly in chronic anemia in ratsman experimental study including ultrastructural, histometric, and stereologic observations. Lab Invest. 1975 Apr;32(4):503–514. [PubMed] [Google Scholar]
- Dillmann E., Johnson D. G., Martin J., Mackler B., Finch C. Catecholamine elevation in iron deficiency. Am J Physiol. 1979 Nov;237(5):R297–R300. doi: 10.1152/ajpregu.1979.237.5.R297. [DOI] [PubMed] [Google Scholar]
- GAFFNEY T. E., BRAUNWALD E. Importance of the adrenergic nervous system in the support of circulatory function in patients with congestive heart failure. Am J Med. 1963 Mar;34:320–324. doi: 10.1016/0002-9343(63)90118-9. [DOI] [PubMed] [Google Scholar]
- GRAETTINGER J. S., PARSONS R. L., CAMPBELL J. A. A correlation of clinical and hemodynamic studies in patients with mild and severe anemia with and without congestive failure. Ann Intern Med. 1963 Apr;58:617–626. doi: 10.7326/0003-4819-58-4-617. [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Mathieu O., Weibel E. R., Krauer R., Lindstedt S. L., Taylor C. R. Design of the mammalian respiratory system. VIII Capillaries in skeletal muscles. Respir Physiol. 1981 Apr;44(1):129–150. doi: 10.1016/0034-5687(81)90080-3. [DOI] [PubMed] [Google Scholar]
- Isoyama S., Grossman W., Wei J. Y. Effect of age on myocardial adaptation to volume overload in the rat. J Clin Invest. 1988 Jun;81(6):1850–1857. doi: 10.1172/JCI113530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoyama S., Wei J. Y., Izumo S., Fort P., Schoen F. J., Grossman W. Effect of age on the development of cardiac hypertrophy produced by aortic constriction in the rat. Circ Res. 1987 Sep;61(3):337–345. doi: 10.1161/01.res.61.3.337. [DOI] [PubMed] [Google Scholar]
- Korecky B., French I. W. Nucleic acid synthesis in enlarged hearts of rats with nutritional anemia. Circ Res. 1967 Nov;21(5):635–640. doi: 10.1161/01.res.21.5.635. [DOI] [PubMed] [Google Scholar]
- LINZBACH A. J. Heart failure from the point of view of quantitative anatomy. Am J Cardiol. 1960 Mar;5:370–382. doi: 10.1016/0002-9149(60)90084-9. [DOI] [PubMed] [Google Scholar]
- Loud A. V., Anversa P. Morphometric analysis of biologic processes. Lab Invest. 1984 Mar;50(3):250–261. [PubMed] [Google Scholar]
- Loud A. V., Beghi C., Olivetti G., Anversa P. Morphometry of right and left ventricular myocardium after strenuous exercise in preconditioned rats. Lab Invest. 1984 Jul;51(1):104–111. [PubMed] [Google Scholar]
- MURRAY J. F., GOLD P., JOHNSON B. L., Jr The circulatory effects of hematocrit variations in normovolemic and hypervolemic dogs. J Clin Invest. 1963 Jul;42:1150–1159. doi: 10.1172/JCI104800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. G., Pfeffer M. A., Pasternak R. C., Markis J. E., Come P. C., Nakao S., Alderman J. D., Ferguson J. J., Safian R. D., Grossman W. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986 Oct;74(4):693–702. doi: 10.1161/01.cir.74.4.693. [DOI] [PubMed] [Google Scholar]
- Oberpriller J. O., Ferrans V. J., Carroll R. J. Changes in DNA content, number of nuclei and cellular dimensions of young rat atrial myocytes in response to left coronary artery ligation. J Mol Cell Cardiol. 1983 Jan;15(1):31–42. doi: 10.1016/0022-2828(83)90305-x. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Anversa P., Loud A. V. Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. II. Tissue composition, capillary growth, and sarcoplasmic alterations. Circ Res. 1980 Apr;46(4):503–512. doi: 10.1161/01.res.46.4.503. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Capasso J. M., Sonnenblick E. H., Anversa P. Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circ Res. 1990 Jul;67(1):23–34. doi: 10.1161/01.res.67.1.23. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Lagrasta C., Quaini F., Ricci R., Moccia G., Capasso J. M., Anversa P. Capillary growth in anemia-induced ventricular wall remodeling in the rat heart. Circ Res. 1989 Nov;65(5):1182–1192. doi: 10.1161/01.res.65.5.1182. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Ricci R., Anversa P. Hyperplasia of myocyte nuclei in long-term cardiac hypertrophy in rats. J Clin Invest. 1987 Dec;80(6):1818–1821. doi: 10.1172/JCI113278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetti G., Ricci R., Lagrasta C., Maniga E., Sonnenblick E. H., Anversa P. Cellular basis of wall remodeling in long-term pressure overload-induced right ventricular hypertrophy in rats. Circ Res. 1988 Sep;63(3):648–657. doi: 10.1161/01.res.63.3.648. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M., Hopkins B. E., Taylor R. R. Regression of left ventricular dilation and hypertrophy after removal of volume overload. Morphological and ultrastructural study. Circ Res. 1974 Jul;35(1):127–135. doi: 10.1161/01.res.35.1.127. [DOI] [PubMed] [Google Scholar]
- Pfeffer M. A., Pfeffer J. M., Frohlich E. D. Pumping ability of the hypertrophying left ventricle of the spontaneously hypertensive rat. Circ Res. 1976 May;38(5):423–429. doi: 10.1161/01.res.38.5.423. [DOI] [PubMed] [Google Scholar]
- Rossi M. A., Carillo S. V., Oliveira J. S. The effect of iron deficiency anemia in the rat on catecholamine levels and heart morphology. Cardiovasc Res. 1981 Jun;15(6):313–319. doi: 10.1093/cvr/15.6.313. [DOI] [PubMed] [Google Scholar]
- SPROULE B. J., MITCHELL J. H., MILLER W. F. Cardiopulmonary physiological responses to heavy exercise in patients with anemia. J Clin Invest. 1960 Feb;39:378–388. doi: 10.1172/JCI104048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spotnitz H. M., Spotnitz W. D., Cottrell T. S., Spiro D., Sonnenblick E. H. Cellular basis for volume related wall thickness changes in the rat left ventricle. J Mol Cell Cardiol. 1974 Aug;6(4):317–331. doi: 10.1016/0022-2828(74)90074-1. [DOI] [PubMed] [Google Scholar]
- Stone H. O., Thompson H. K., Jr, Schmidt-Nielsen K. Influence of erythrocytes on blood viscosity. Am J Physiol. 1968 Apr;214(4):913–918. doi: 10.1152/ajplegacy.1968.214.4.913. [DOI] [PubMed] [Google Scholar]
- Varat M. A., Adolph R. J., Fowler N. O. Cardiovascular effects of anemia. Am Heart J. 1972 Mar;83(3):415–426. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- Weisman H. F., Bush D. E., Mannisi J. A., Weisfeldt M. L., Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation. 1988 Jul;78(1):186–201. doi: 10.1161/01.cir.78.1.186. [DOI] [PubMed] [Google Scholar]



