Abstract
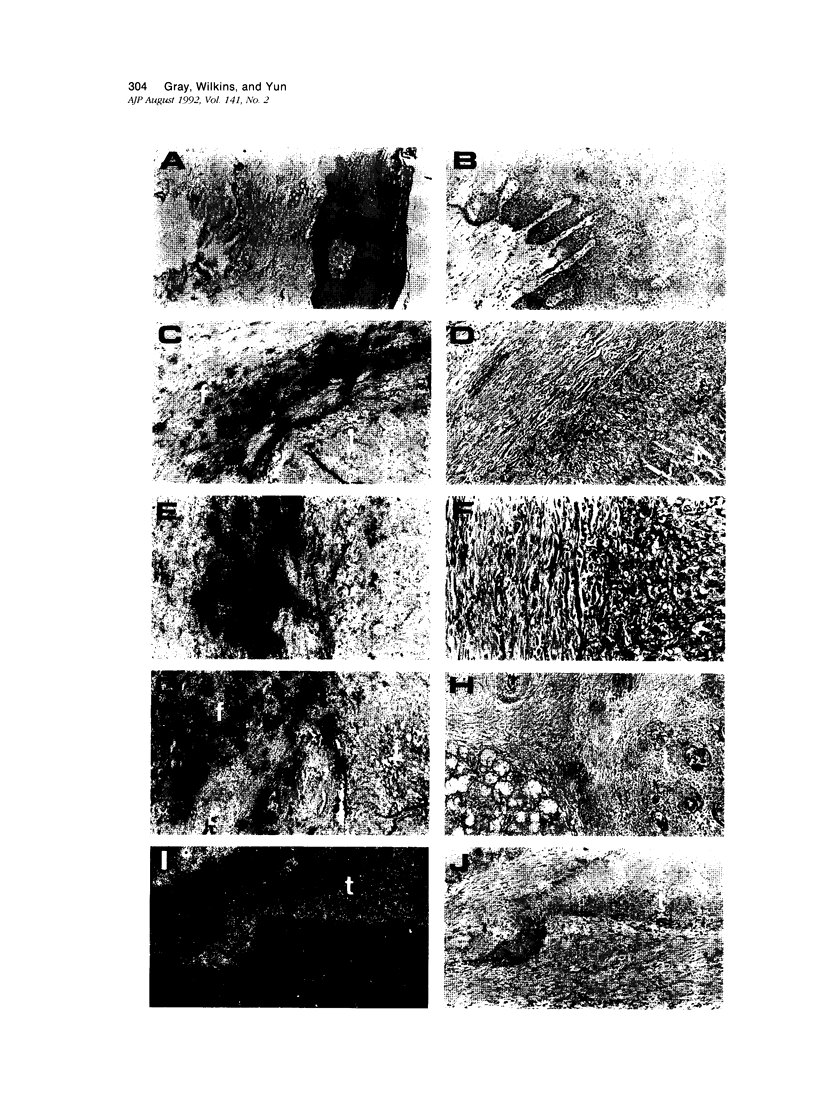
In this study, in situ hybridization techniques were used to determine the location of interstitial collagenase and tissue inhibitor of metalloproteinase (TIMP) gene expression in samples from 11 squamous cell carcinomas of the head and neck (particularly the oral cavity) and from non-neoplastic mucosa of the same region. Ten of the 11 carcinomas examined showed abundant levels of collagenase gene expression in stromal fibroblasts within connective tissues immediately adjacent to tumor masses. Lower levels were detected in basaloid tumor cells located at the periphery of several tumor masses. Interstitial collagenase expression was consistently low in all normal, hyperplastic, and dysplastic epithelial sections. TIMP gene expression was negligible in all tissues examined. These results support the view that stromal interstitial collagenase production may play a key role in assisting invasiveness of squamous cell carcinoma of the head and neck.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson M., Schiling R. W., Huang C. C., Salome R. G. Collagenase activity in epidermoid carcinoma of the oral cavity and larynx. Ann Otol Rhinol Laryngol. 1975 Mar-Apr;84(2 Pt 1):158–163. doi: 10.1177/000348947508400203. [DOI] [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Gordon J. M., Reddick M. E., Eisen A. Z. Quantitation and immunocytochemical localization of human skin collagenase in basal cell carcinoma. J Invest Dermatol. 1977 Oct;69(4):363–367. doi: 10.1111/1523-1747.ep12510240. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Uitto J., Walters R. C., Eisen A. Z. Enhanced collagenase production by fibroblasts derived from human basal cell carcinomas. Cancer Res. 1979 Nov;39(11):4594–4599. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Biswas C., Nugent M. A. Membrane association of collagenase stimulatory factor(s) from B-16 melanoma cells. J Cell Biochem. 1987 Nov;35(3):247–258. doi: 10.1002/jcb.240350307. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., McMillan R. M., Fahey J. V., Harris E. D., Jr Collagenase production by synovial fibroblasts treated with phorbol myristate acetate. Arthritis Rheum. 1979 Oct;22(10):1109–1116. doi: 10.1002/art.1780221010. [DOI] [PubMed] [Google Scholar]
- Burman J. F., Carter R. L. Lysis of type-I collagen by squamous carcinomas of the head and neck. Int J Cancer. 1985 Jul 15;36(1):109–116. doi: 10.1002/ijc.2910360117. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Ellis S. M., Nabeshima K., Biswas C. Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res. 1989 Jun 15;49(12):3385–3391. [PubMed] [Google Scholar]
- Flenniken A. M., Williams B. R. Developmental expression of the endogenous TIMP gene and a TIMP-lacZ fusion gene in transgenic mice. Genes Dev. 1990 Jul;4(7):1094–1106. doi: 10.1101/gad.4.7.1094. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Golde D. W., Kaufman S. E., Westbrook C. A., Hewick R. M., Kaufman R. J., Wong G. G., Temple P. A., Leary A. C., Brown E. L. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. 1985 Jun 27-Jul 3Nature. 315(6022):768–771. doi: 10.1038/315768a0. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Khokha R., Waterhouse P., Yagel S., Lala P. K., Overall C. M., Norton G., Denhardt D. T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989 Feb 17;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima K., Lane W. S., Biswas C. Partial sequencing and characterization of the tumor cell-derived collagenase stimulatory factor. Arch Biochem Biophys. 1991 Feb 15;285(1):90–96. doi: 10.1016/0003-9861(91)90332-d. [DOI] [PubMed] [Google Scholar]
- O'Grady R. L., Upfold L. I., Stephens R. W. Rat mammary carcinoma cells secrete active collagenase and activate latent enzyme in the stroma via plasminogen activator. Int J Cancer. 1981 Oct 15;28(4):509–515. doi: 10.1002/ijc.2910280418. [DOI] [PubMed] [Google Scholar]
- Okada Y., Watanabe S., Nakanishi I., Kishi J., Hayakawa T., Watorek W., Travis J., Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988 Feb 29;229(1):157–160. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Wilhelm S. M., Eisen A. Z., Teter M., Clark S. D., Kronberger A., Goldberg G. Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3756–3760. doi: 10.1073/pnas.83.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]