Abstract
Manganese-containing superoxide dismutase (Mn-SOD) is a mitochondrial enzyme implicated in cellular defense from oxidative damage. We investigated the immunocytochemical distribution and protein concentration of Mn-SOD in rat lungs in response to aerosolized crocidolite asbestos or cristobalite silica, fibrogenic minerals eliciting generation of oxidants by cellular and acellular pathways. Rats were exposed to 7-10 mg/m3 dust for 6 hours a day for 10 days. Experimental and sham control rats were euthanized 10 days after cessation of exposure, and lungs prepared for immunocytochemistry and determination of amounts of Mn-SOD protein. Quantitation of Western blots showed that the amount of immunodetectable Mn-SOD increased in lungs exposed to asbestos or silica by approximately 1.3- and 2.4-fold, respectively, when compared with sham controls. Immunoelectron microscopy using the protein A-gold technique showed that Mn-SOD was located predominantly in mitochondria of type II epithelial cells. Fibroblasts contained little immunodetectable Mn-SOD, whereas type I epithelial cells, polymorphonuclear leukocytes (PMNs), and endothelial cells contained no detectable protein. Some alveolar macrophages (AMs) were found with labeled mitochondria, whereas most interstitial macrophages had no detectable protein. Quantitative analysis of type II cells showed that the number of immunogold particles per unit of mitochondrial area increased in the terminal airways of lungs exposed to asbestos or silica by 2.2-fold and 3.6-fold, respectively, over controls. Morphometric analyses indicated that the size of type II cells, as well as the number of interstitial macrophages and PMNs, increased in the terminal respiratory tissue of silica-exposed lungs. Less pronounced histopathologic changes were evident in asbestos-exposed lungs. These results indicate that the relative concentration of Mn-SOD increases preferentially in type II epithelial cells subsequent to inhalation of silica or asbestos. The magnitude of induction of Mn-SOD protein in these cells and whole lung correlated with the inflammatory potential of these minerals.
Full text
PDF


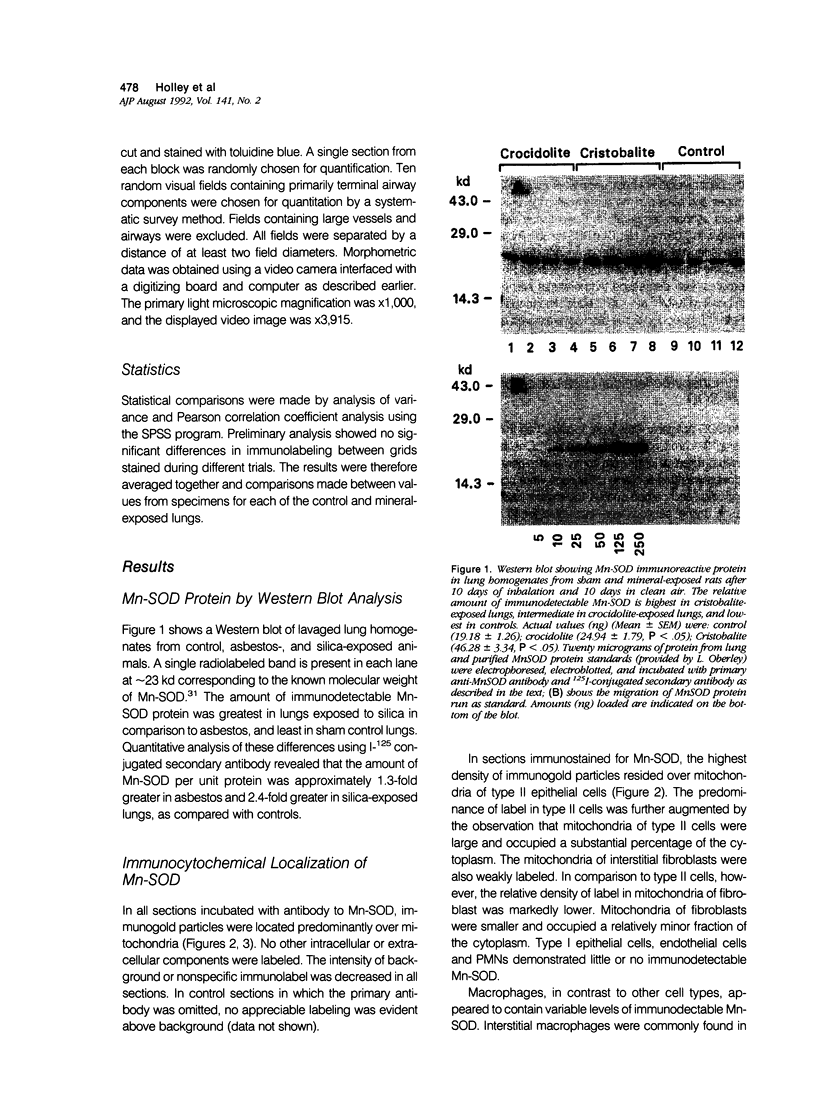
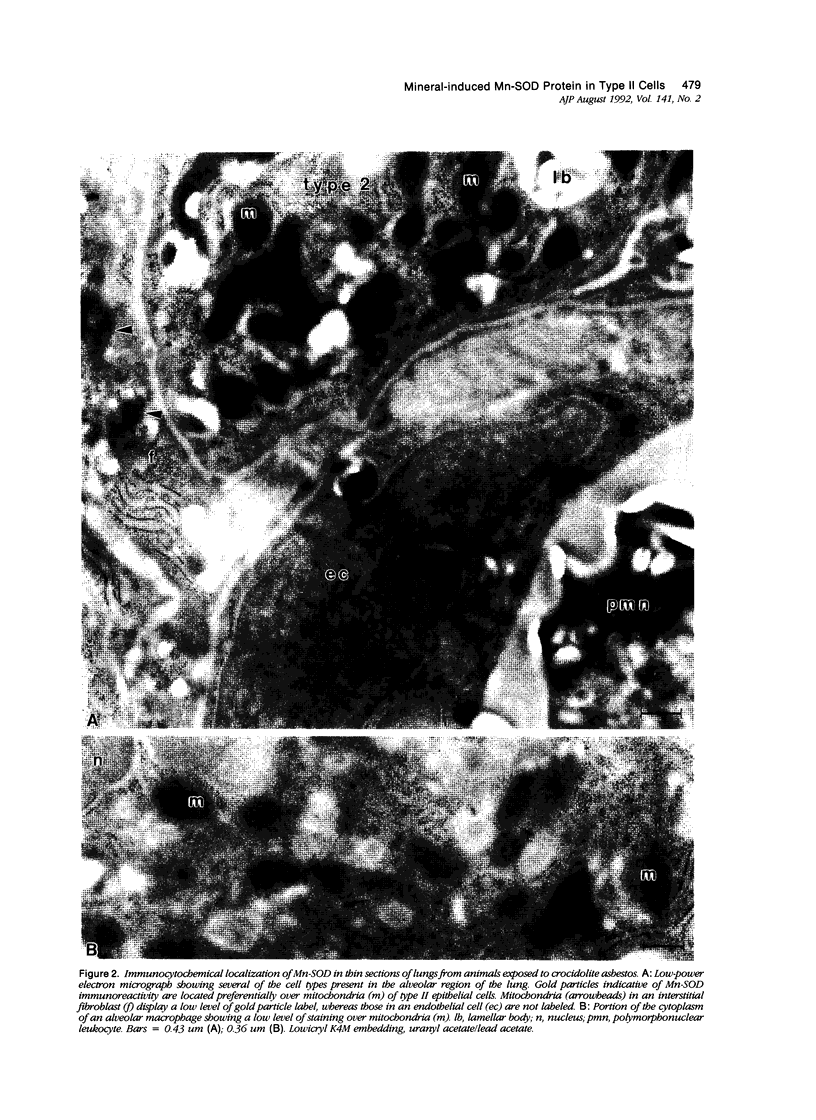
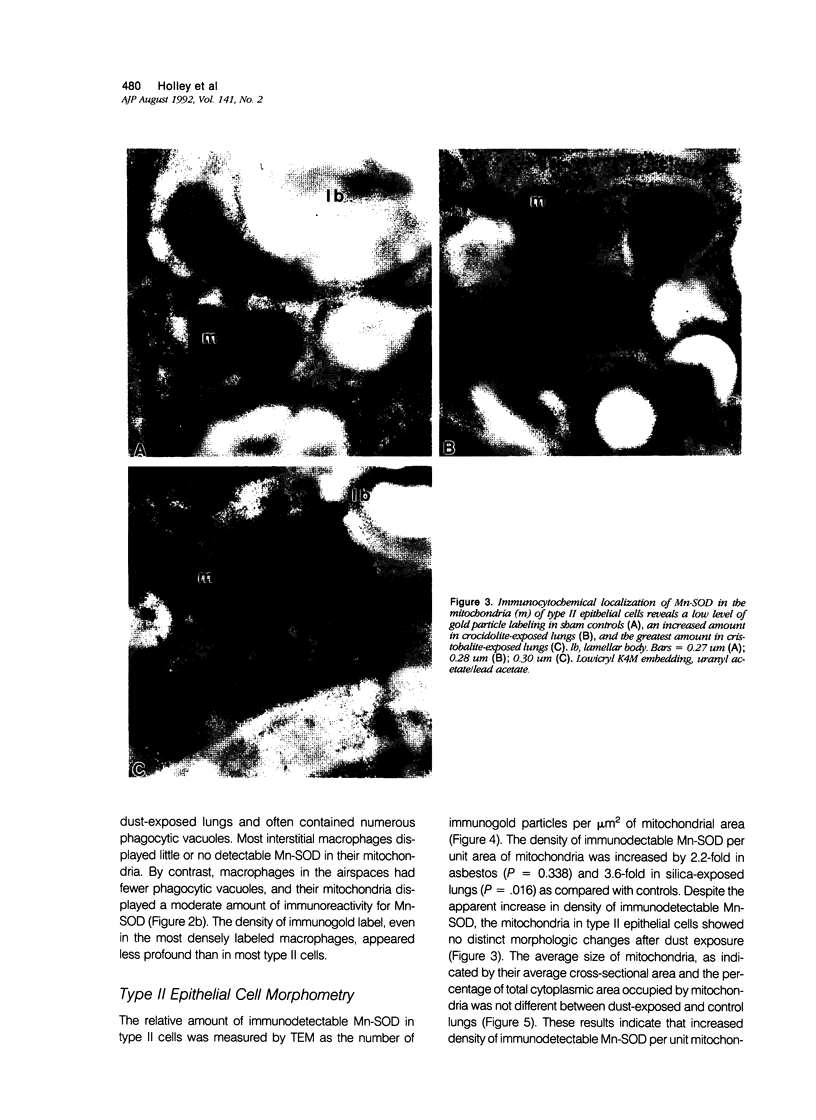
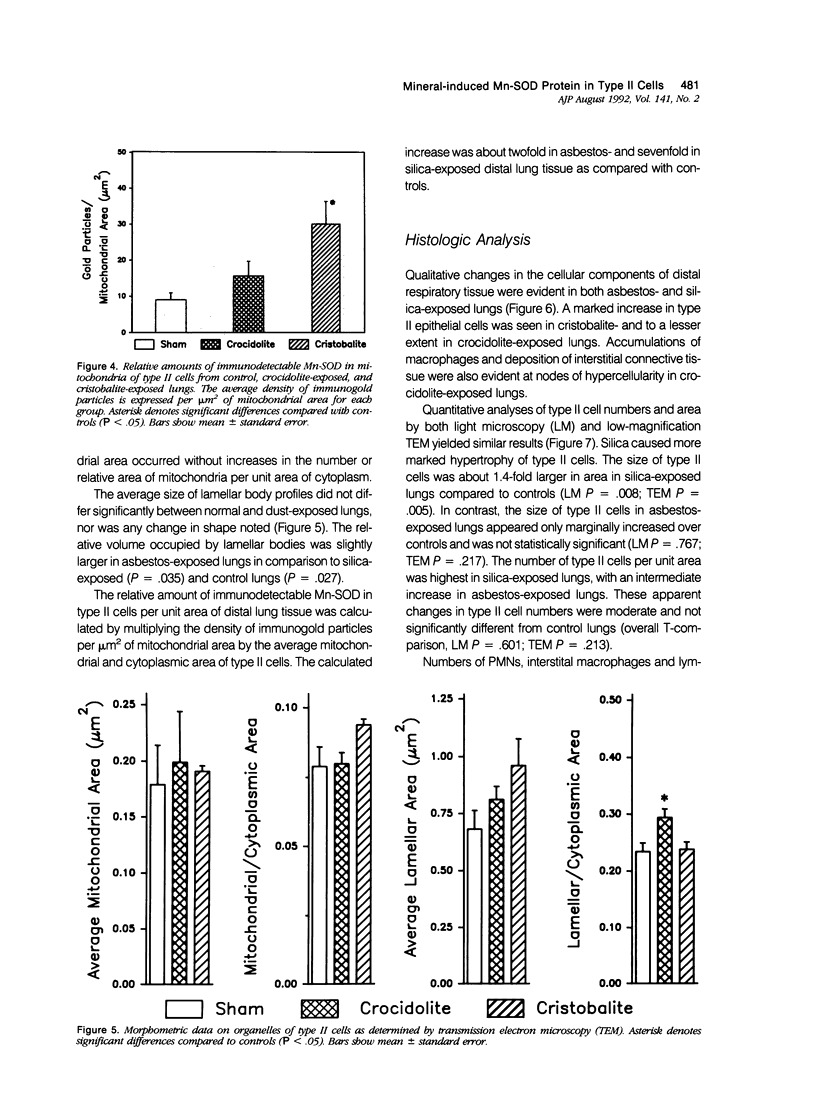

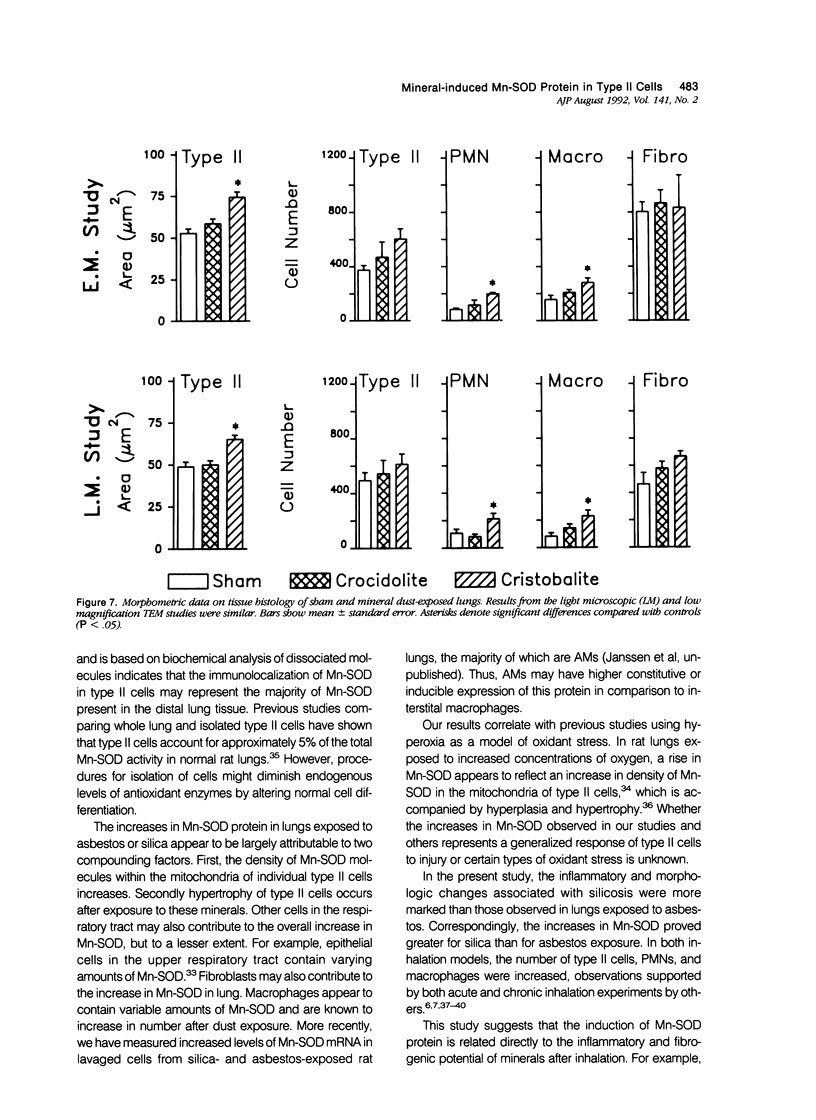


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absher M. P., Trombley L., Hemenway D. R., Mickey R. M., Leslie K. O. Biphasic cellular and tissue response of rat lungs after eight-day aerosol exposure to the silicon dioxide cristobalite. Am J Pathol. 1989 Jun;134(6):1243–1251. [PMC free article] [PubMed] [Google Scholar]
- Barry B. E., Wong K. C., Brody A. R., Crapo J. D. Reaction of rat lungs to inhaled chrysotile asbestos following acute and subchronic exposures. Exp Lung Res. 1983 Jul;5(1):1–21. doi: 10.3109/01902148309061501. [DOI] [PubMed] [Google Scholar]
- Bozelka B. E., Sestini P., Gaumer H. R., Hammad Y., Heather C. J., Salvaggio J. E. A murine model of asbestosis. Am J Pathol. 1983 Sep;112(3):326–337. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang L. Y., Overby L. H., Brody A. R., Crapo J. D. Progressive lung cell reactions and extracellular matrix production after a brief exposure to asbestos. Am J Pathol. 1988 Apr;131(1):156–170. [PMC free article] [PubMed] [Google Scholar]
- Donaldson K., Slight J., Bolton R. E. Oxidant production by control and inflammatory bronchoalveolar leukocyte populations treated with mineral dusts in vitro. Inflammation. 1988 Jun;12(3):231–243. doi: 10.1007/BF00920075. [DOI] [PubMed] [Google Scholar]
- Eberhardt M. K., Román-Franco A. A., Quiles M. R. Asbestos-induced decomposition of hydrogen peroxide. Environ Res. 1985 Aug;37(2):287–292. doi: 10.1016/0013-9351(85)90108-2. [DOI] [PubMed] [Google Scholar]
- Edmondson S. W., Wu R., Mossman B. T. Regulation of differentiation and keratin protein expression by vitamin A in primary cultures of hamster tracheal epithelial cells. J Cell Physiol. 1990 Jan;142(1):21–30. doi: 10.1002/jcp.1041420104. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Mason R. J., Williams M. C., Crapo J. D. Antioxidant enzyme activity in alveolar type II cells after exposure of rats to hyperoxia. Exp Lung Res. 1986;10(2):203–222. doi: 10.3109/01902148609061493. [DOI] [PubMed] [Google Scholar]
- Hansen K., Mossman B. T. Generation of superoxide (O2-.) from alveolar macrophages exposed to asbestiform and nonfibrous particles. Cancer Res. 1987 Mar 15;47(6):1681–1686. [PubMed] [Google Scholar]
- Heffner J. E., Repine J. E. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989 Aug;140(2):531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Hemenway D. R., MacAskill S. M. Design, development and test results of a horizontal flow inhalation toxicology facility. Am Ind Hyg Assoc J. 1982 Dec;43(12):874–879. doi: 10.1080/15298668291410738. [DOI] [PubMed] [Google Scholar]
- Janssen Y. M., Marsh J. P., Absher M. P., Hemenway D., Vacek P. M., Leslie K. O., Borm P. J., Mossman B. T. Expression of antioxidant enzymes in rat lungs after inhalation of asbestos or silica. J Biol Chem. 1992 May 25;267(15):10625–10630. [PubMed] [Google Scholar]
- Janssen Y. M., Marsh J. P., Absher M., Borm P. J., Mossman B. T. Increases in endogenous antioxidant enzymes during asbestos inhalation in rats. Free Radic Res Commun. 1990;11(1-3):53–58. doi: 10.3109/10715769009109667. [DOI] [PubMed] [Google Scholar]
- Kagan E., Oghiso Y., Hartmann D. P. The effects of chrysotile and crocidolite asbestos on the lower respiratory tract: analysis of bronchoalveolar lavage constituents. Environ Res. 1983 Dec;32(2):382–397. doi: 10.1016/0013-9351(83)90120-2. [DOI] [PubMed] [Google Scholar]
- Low R. B., Leslie K. O., Hemenway D. R., Absher M., Adler K. B., Giancola M. S., Vacek P. M. Alveolar type II cell response in rats exposed to aerosols of alpha-cristobalite. Am J Pathol. 1990 Apr;136(4):923–931. [PMC free article] [PubMed] [Google Scholar]
- Mossman B. T., Marsh J. P. Evidence supporting a role for active oxygen species in asbestos-induced toxicity and lung disease. Environ Health Perspect. 1989 May;81:91–94. doi: 10.1289/ehp.898191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman B. T., Marsh J. P., Sesko A., Hill S., Shatos M. A., Doherty J., Petruska J., Adler K. B., Hemenway D., Mickey R. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1266–1271. doi: 10.1164/ajrccm/141.5_Pt_1.1266. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Oberley L. W., Slattery A. F., Lauchner L. J., Elwell J. H. Immunohistochemical localization of antioxidant enzymes in adult Syrian hamster tissues and during kidney development. Am J Pathol. 1990 Jul;137(1):199–214. [PMC free article] [PubMed] [Google Scholar]
- Ono K., Kimura S., Nakano M., Naruse T. Detection of heterogeneity of Cu, Zn-superoxide dismutase with monoclonal antibodies and the establishment of a highly sensitive fluorescence sandwich enzyme-linked immunosorbent assay. FEBS Lett. 1991 Apr 22;282(1):115–118. doi: 10.1016/0014-5793(91)80457-e. [DOI] [PubMed] [Google Scholar]
- Panus P. C., Shearer J., Freeman B. A. Pulmonary metabolism of reactive oxygen species. Exp Lung Res. 1988;14 (Suppl):959–976. doi: 10.3109/01902148809064186. [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Young S. L., Fram E. K., Crapo J. D. Alveolar type II cell responses to chronic inhalation of chrysotile asbestos in rats. Am J Respir Cell Mol Biol. 1990 Dec;3(6):543–552. doi: 10.1165/ajrcmb/3.6.543. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Shull S., Heintz N. H., Periasamy M., Manohar M., Janssen Y. M., Marsh J. P., Mossman B. T. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991 Dec 25;266(36):24398–24403. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Freeman B. A., Crapo J. D. Intracellular localization of the copper-zinc and manganese superoxide dismutases in rat liver parenchymal cells. Lab Invest. 1986 Sep;55(3):363–371. [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of mangano superoxide dismutase from Escherichia coli B. J Biol Chem. 1978 Dec 25;253(24):8708–8720. [PubMed] [Google Scholar]
- Taatjes D. J., Barcomb L. A., Leslie K. O., Low R. B. Lectin binding patterns to terminal sugars of rat lung alveolar epithelial cells. J Histochem Cytochem. 1990 Feb;38(2):233–244. doi: 10.1177/38.2.1688898. [DOI] [PubMed] [Google Scholar]
- Visner G. A., Dougall W. C., Wilson J. M., Burr I. A., Nick H. S. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor. Role in the acute inflammatory response. J Biol Chem. 1990 Feb 15;265(5):2856–2864. [PubMed] [Google Scholar]
- Voisin C., Aerts C., Wallaert B. Prevention of in vitro oxidant-mediated alveolar macrophage injury by cellular glutathione and precursors. Bull Eur Physiopathol Respir. 1987 Jul-Aug;23(4):309–313. [PubMed] [Google Scholar]
- Weitzman S. A., Graceffa P. Asbestos catalyzes hydroxyl and superoxide radical generation from hydrogen peroxide. Arch Biochem Biophys. 1984 Jan;228(1):373–376. doi: 10.1016/0003-9861(84)90078-x. [DOI] [PubMed] [Google Scholar]
- White C. W., Repine J. E. Pulmonary antioxidant defense mechanisms. Exp Lung Res. 1985;8(2-3):81–96. doi: 10.3109/01902148509057515. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]