Abstract
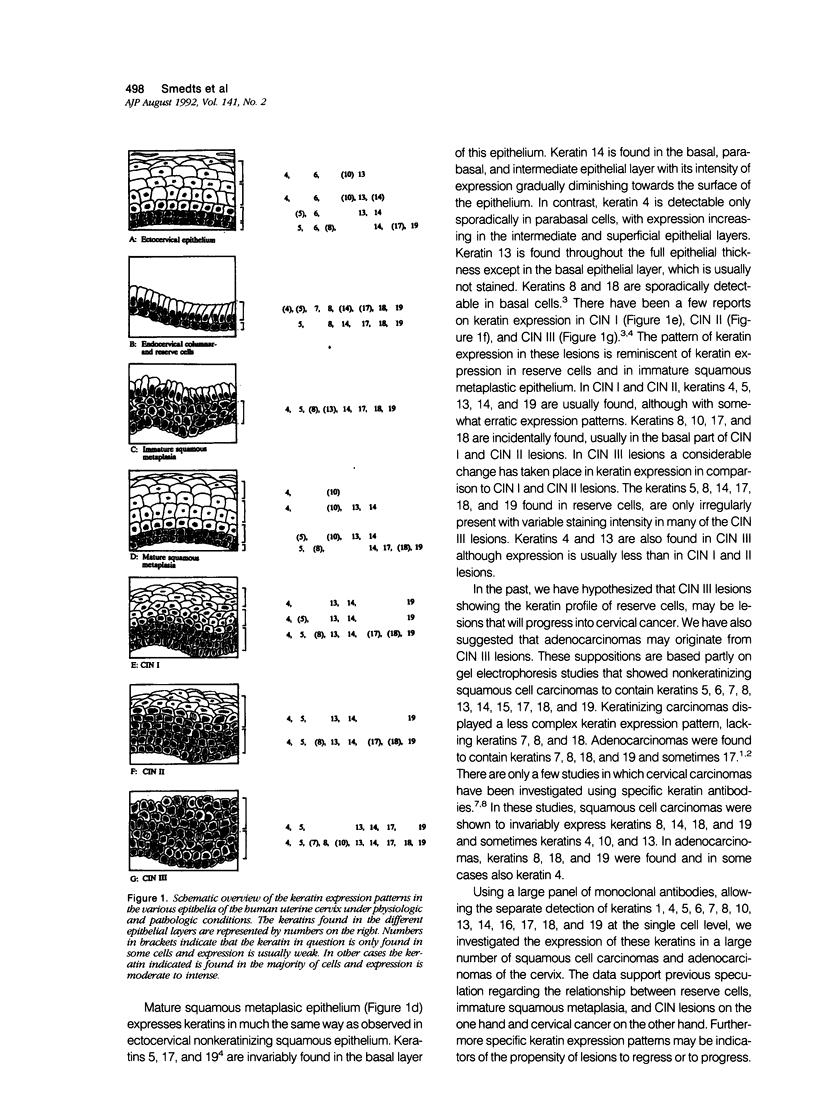
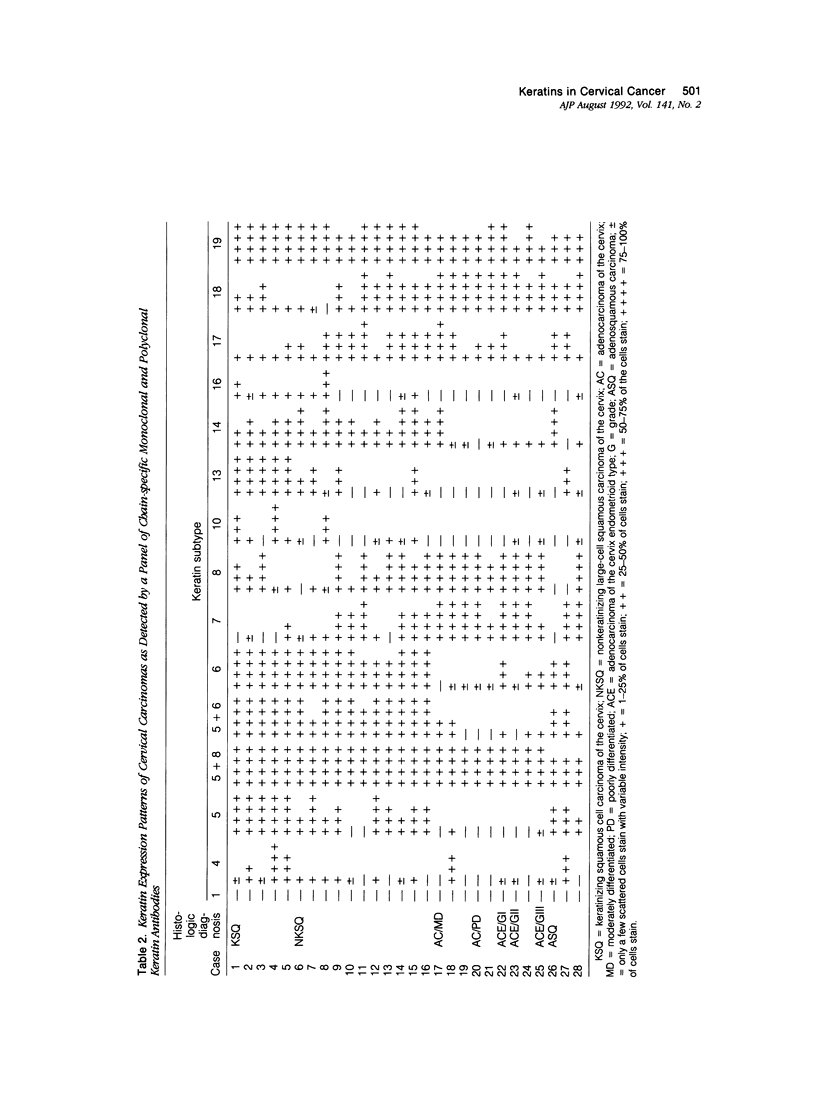
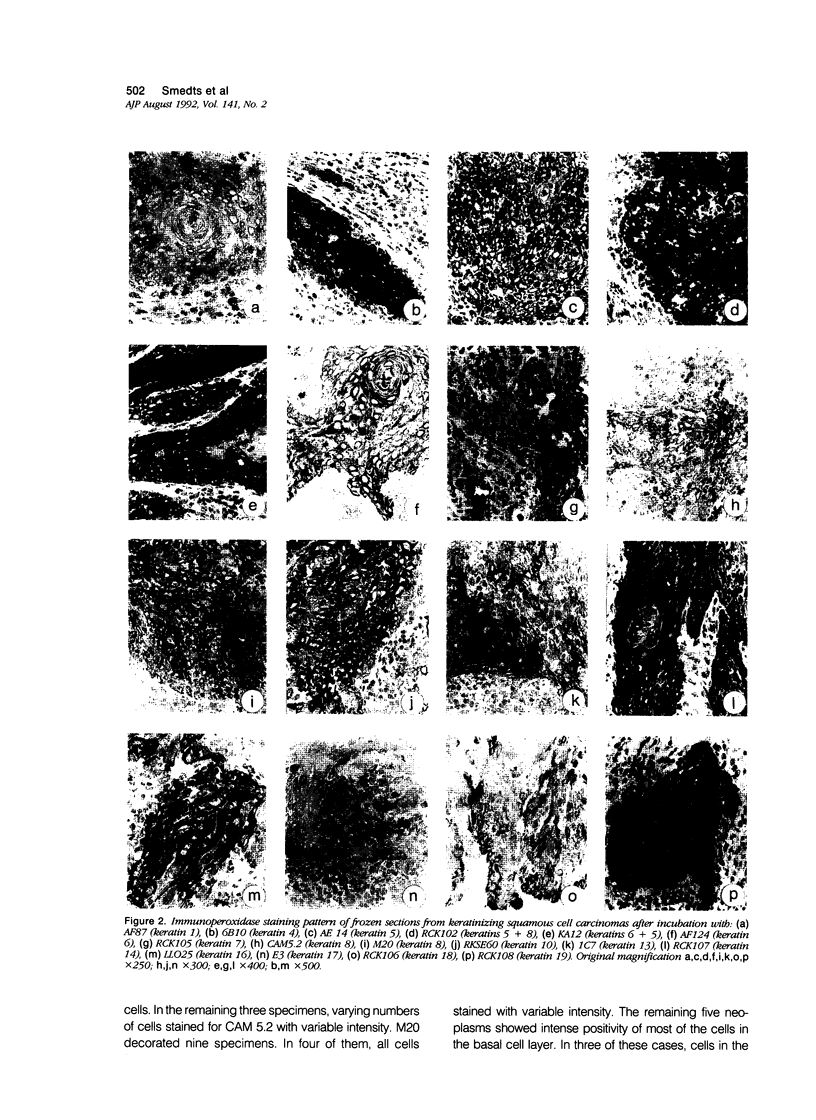
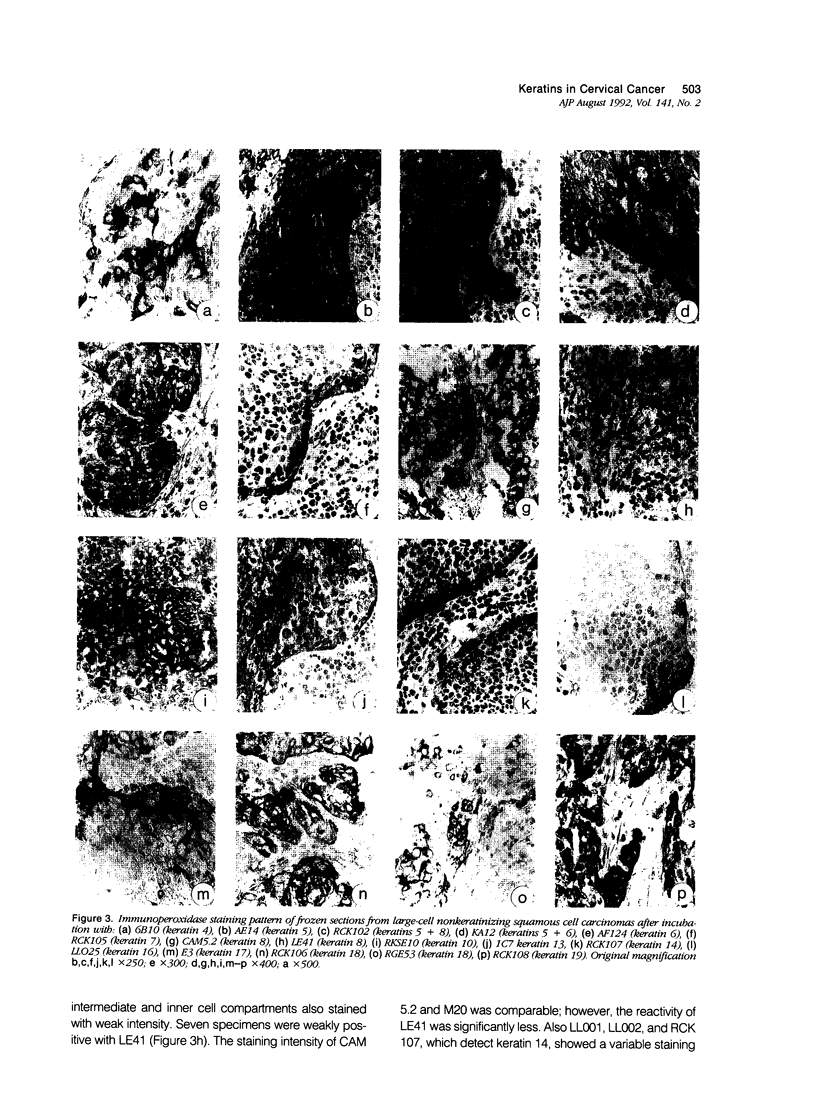
Using a panel of 21 monoclonal and 2 polyclonal keratin antibodies, capable of detecting separately 11 subtypes of their epithelial intermediate filament proteins at the single cell level, we investigated keratin expression in 16 squamous cell carcinomas, 9 adenocarcinomas, and 3 adenosquamous carcinomas of the human uterine cervix. The keratin phenotype of the keratinizing squamous cell carcinoma was found to be most complex comprising keratins 4, 5, 6, 8, 13, 14, 16, 17, 18, 19, and usually keratin 10. The nonkeratinizing variety of the squamous cell carcinoma expressed keratins 6, 14, 17, and 19 in all cases, usually 4, 5, 7, 8, and 18, and sometimes keratins 10, 13, and 16. Adenocarcinomas displayed a less complex keratin expression pattern comprising keratins 7, 8, 17, 18, and 19, while keratin 14 was often present and keratins 4, 5, 10 and 13 were sporadically found in individual cells in a few cases. These keratin phenotypes may be useful in differential diagnostic considerations when distinguishing between keratinizing and nonkeratinizing carcinomas (using keratin 10, 13, and 16 antibodies), and also in the distinction between nonkeratinizing carcinomas and poorly differentiated adenocarcinomas, which do not express keratins 5 and 6. Keratin 17 may also be useful in distinguishing carcinomas of the cervix from those of the colon and also from mesotheliomas. Furthermore the presence of keratin 17 in a CIN I, II, or III lesion may indicate progressive potential while its absence could be indicative of a regressive behavior. Because most carcinomas express keratins 8, 14, 17, 18, and 19, we propose that this expression pattern reflects the origin of cervical cancer from a common progenitor cell, i.e., the endocervical reserve cell that has been shown to express keratins 5, 8, 14, 17, 18, and 19.
Full text
PDF



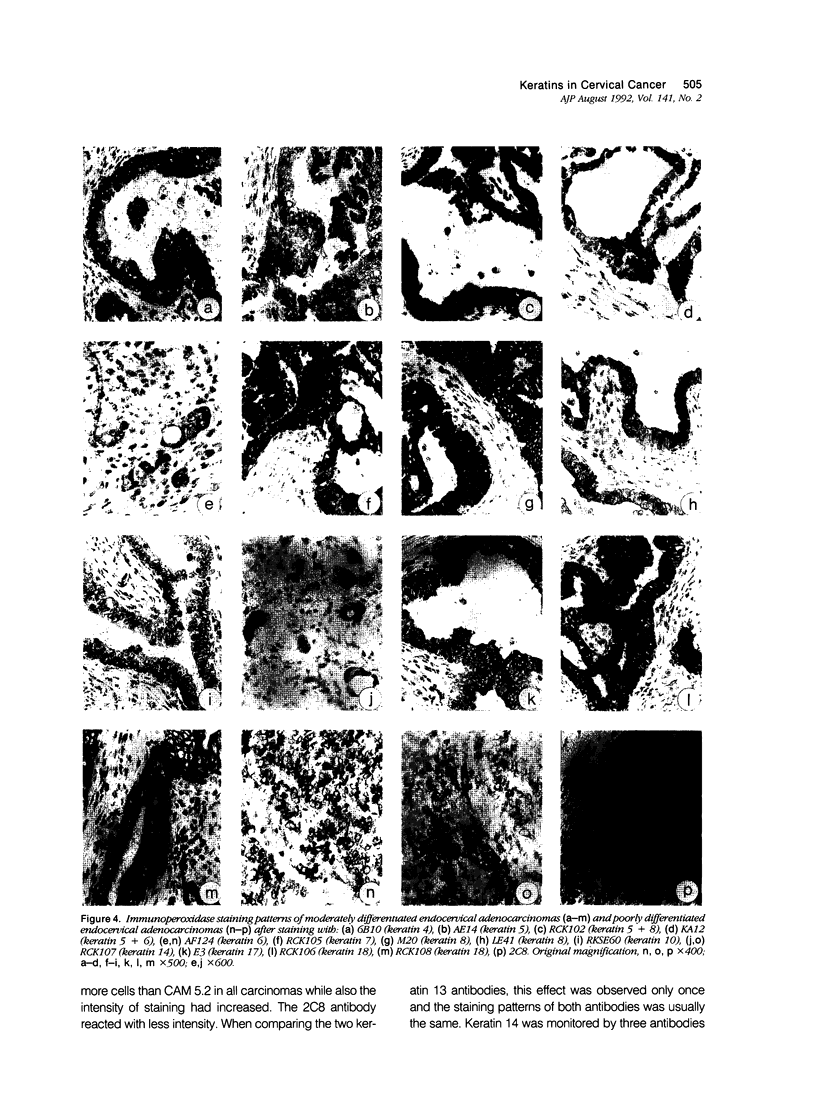
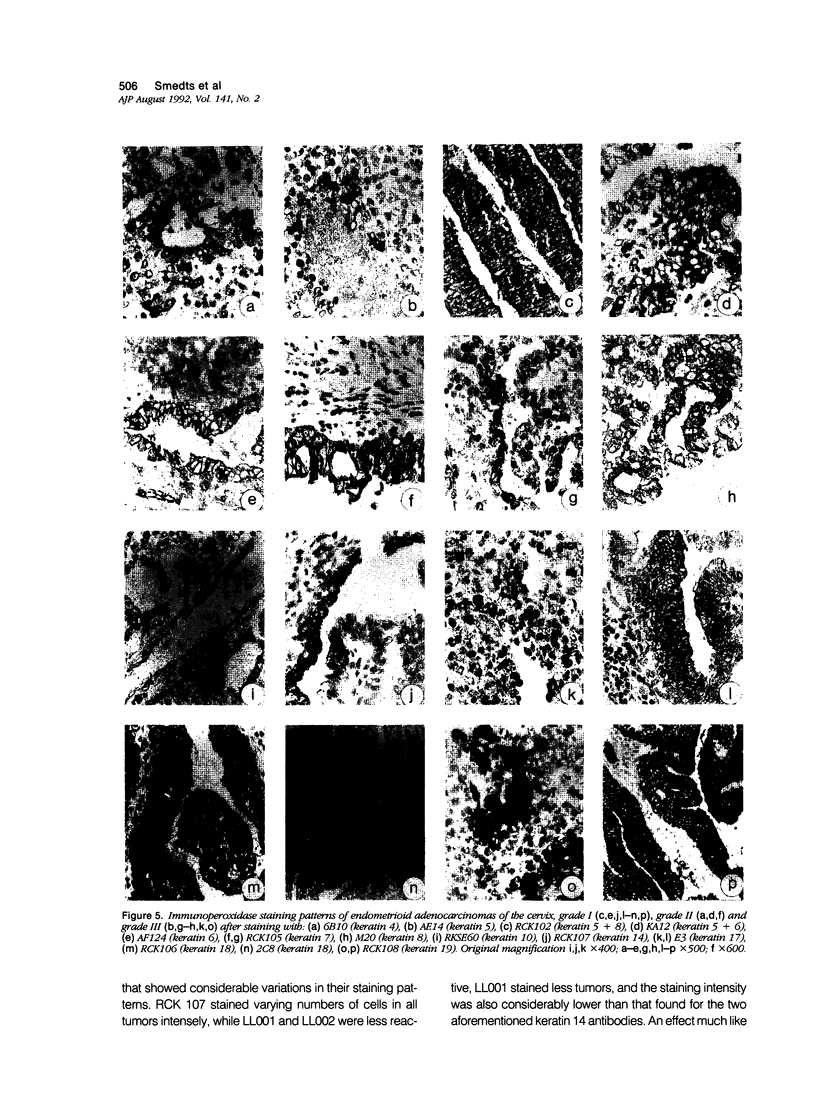





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B., Kiberu S., Purvis J., Wilkinson L., Horne C. H. Cytokeratins in cervical dysplasia and neoplasia: a comparative study of immunohistochemical staining using monoclonal antibodies NCL-5D3, CAM 5.2, and PKK1. J Pathol. 1988 May;155(1):71–75. doi: 10.1002/path.1711550111. [DOI] [PubMed] [Google Scholar]
- Broers J. L., Carney D. N., Klein Rot M., Schaart G., Lane E. B., Vooijs G. P., Ramaekers F. C. Intermediate filament proteins in classic and variant types of small cell lung carcinoma cell lines: a biochemical and immunochemical analysis using a panel of monoclonal and polyclonal antibodies. J Cell Sci. 1986 Jul;83:37–60. doi: 10.1242/jcs.83.1.37. [DOI] [PubMed] [Google Scholar]
- Broers J. L., Ramaekers F. C., Rot M. K., Oostendorp T., Huysmans A., van Muijen G. N., Wagenaar S. S., Vooijs G. P. Cytokeratins in different types of human lung cancer as monitored by chain-specific monoclonal antibodies. Cancer Res. 1988 Jun 1;48(11):3221–3229. [PubMed] [Google Scholar]
- Czernobilsky B., Moll R., Franke W. W., Dallenbach-Hellweg G., Hohlweg-Majert P. Intermediate filaments of normal and neoplastic tissues of the female genital tract with emphasis on problems of differential tumor diagnosis. Pathol Res Pract. 1984 Sep;179(1):31–37. doi: 10.1016/S0344-0338(84)80058-8. [DOI] [PubMed] [Google Scholar]
- Dallenbach-Hellweg G., Lang G. Immunohistochemical studies on uterine tumors. I. Invasive squamous cell carcinomas of the cervix and their precursors. Pathol Res Pract. 1991 Jan;187(1):36–43. doi: 10.1016/S0344-0338(11)81042-3. [DOI] [PubMed] [Google Scholar]
- Gigi-Leitner O., Geiger B., Levy R., Czernobilsky B. Cytokeratin expression in squamous metaplasia of the human uterine cervix. Differentiation. 1986;31(3):191–205. doi: 10.1111/j.1432-0436.1986.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Ivanyi D., Groeneveld E., Van Doornewaard G., Mooi W. J., Hageman P. C. Keratin subtypes in carcinomas of the uterine cervix: implications for histogenesis and differential diagnosis. Cancer Res. 1990 Aug 15;50(16):5143–5152. [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh I. M., Pulford K. A., Ramaekers F. C., Lane E. B. Psoriasis: maintenance of an intact monolayer basal cell differentiation compartment in spite of hyperproliferation. Br J Dermatol. 1985 Jul;113(1):53–64. doi: 10.1111/j.1365-2133.1985.tb02044.x. [DOI] [PubMed] [Google Scholar]
- Moll R., Dhouailly D., Sun T. T. Expression of keratin 5 as a distinctive feature of epithelial and biphasic mesotheliomas. An immunohistochemical study using monoclonal antibody AE14. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):129–145. doi: 10.1007/BF02890064. [DOI] [PubMed] [Google Scholar]
- Moll R., Levy R., Czernobilsky B., Hohlweg-Majert P., Dallenbach-Hellweg G., Franke W. W. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Invest. 1983 Nov;49(5):599–610. [PubMed] [Google Scholar]
- Purkis P. E., Steel J. B., Mackenzie I. C., Nathrath W. B., Leigh I. M., Lane E. B. Antibody markers of basal cells in complex epithelia. J Cell Sci. 1990 Sep;97(Pt 1):39–50. doi: 10.1242/jcs.97.1.39. [DOI] [PubMed] [Google Scholar]
- Puts J. J., Moesker O., Kenemans P., Vooijs G. P., Ramaekers F. C. Expression of cytokeratins in early neoplastic epithelial lesions of the uterine cervix. Int J Gynecol Pathol. 1985;4(4):300–313. doi: 10.1097/00004347-198512000-00003. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Schiller D. L., Hatzfeld M., Achtstätter T., Moll R., Jorcano J. L., Magin T. M., Franke W. W. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Moesker O., Kant A., Jap P., Herman C., Vooijs P. Monoclonal antibody to keratin filaments, specific for glandular epithelia and their tumors. Use in surgical pathology. Lab Invest. 1983 Sep;49(3):353–361. [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., van Niekerk C., Poels L., Schaafsma E., Huijsmans A., Robben H., Schaart G., Vooijs P. Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas. Am J Pathol. 1990 Mar;136(3):641–655. [PMC free article] [PubMed] [Google Scholar]
- Reagan J. W., Fu Y. S. Histologic types and prognosis of cancers of the uterine cervix. Int J Radiat Oncol Biol Phys. 1979 Jul;5(7):1015–1020. doi: 10.1016/0360-3016(79)90611-4. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Cheng C. K., Titterington L., Meyers C. A., Stanley J. R., Steinert P. M., Yuspa S. H. Synthetic peptides corresponding to keratin subunits elicit highly specific antibodies. J Biol Chem. 1984 Jul 10;259(13):8037–8040. [PubMed] [Google Scholar]
- Schaafsma H. E., Ramaekers F. C., van Muijen G. N., Lane E. B., Leigh I. M., Robben H., Huijsmans A., Ooms E. C., Ruiter D. J. Distribution of cytokeratin polypeptides in human transitional cell carcinomas, with special emphasis on changing expression patterns during tumor progression. Am J Pathol. 1990 Feb;136(2):329–343. [PMC free article] [PubMed] [Google Scholar]
- Schaafsma H. E., Ramaekers F. C., van Muijen G. N., Ooms E. C., Ruiter D. J. Distribution of cytokeratin polypeptides in epithelia of the adult human urinary tract. Histochemistry. 1989;91(2):151–159. doi: 10.1007/BF00492389. [DOI] [PubMed] [Google Scholar]
- Smedts F., Ramaekers F., Robben H., Pruszczynski M., van Muijen G., Lane B., Leigh I., Vooijs P. Changing patterns of keratin expression during progression of cervical intraepithelial neoplasia. Am J Pathol. 1990 Mar;136(3):657–668. [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky S. M., Guelstein V. I., Tchipysheva T. A., Krutovskikh V. A., Bannikov G. A. Patterns of expression of keratin 17 in human epithelia: dependency on cell position. J Cell Sci. 1989 Jul;93(Pt 3):419–426. doi: 10.1242/jcs.93.3.419. [DOI] [PubMed] [Google Scholar]
- Wetzels R. H., Kuijpers H. J., Lane E. B., Leigh I. M., Troyanovsky S. M., Holland R., van Haelst U. J., Ramaekers F. C. Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol. 1991 Mar;138(3):751–763. [PMC free article] [PubMed] [Google Scholar]
- Wetzels R. H., Schaafsma H. E., Leigh I. M., Lane E. B., Troyanovsky S. M., Wagenaar S. S., Vooijs G. P., Ramaekers F. C. Laminin and type VII collagen distribution in different types of human lung carcinoma: correlation with expression of keratins 14, 16, 17 and 18. Histopathology. 1992 Apr;20(4):295–303. doi: 10.1111/j.1365-2559.1992.tb00986.x. [DOI] [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., Franke W. W., Achtstätter T., Haasnoot W. H., Ponec M., Warnaar S. O. Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res. 1986 Jan;162(1):97–113. doi: 10.1016/0014-4827(86)90429-5. [DOI] [PubMed] [Google Scholar]







