Abstract
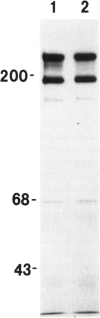
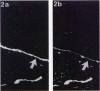
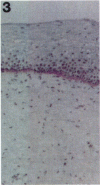
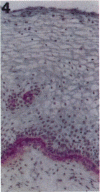
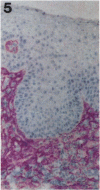
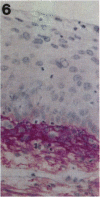
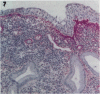
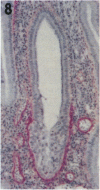
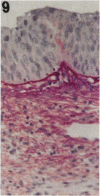
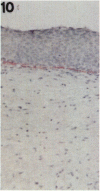
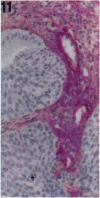
Tenascin is an extracellular matrix glycoprotein that is widely expressed during embryogenesis. In adults, it is restricted to select sites, including certain epithelial-stromal interfaces, but is notably enhanced in active inflammatory-reactive processes and in the stroma of many neoplasms. The authors immunostained with the monoclonal antibody (MAb) 100EB2 cryosections of vulvar and cervical biopsies displaying convincing koilocytosis with variable degrees of hyperplasia-dysplasia; in situ carcinomas were included. The presence of human papillomaviruses (HPV) 6, 11, 16, 18, 31, or 33 was confirmed by in situ hybridization in a subset of cases. Findings were compared with normal controls. The study was extended with the MAb 143DB7 that reacted with tenascin in paraffin sections. In normal samples, tenascin immunoreaction appeared as a delicate, continuous rim, in the immediate vicinity of the laminin staining; in parakeratotic areas, the rim was thicker. In foci of hyperplastic-dysplastic epithelium with or without koilocytosis, a distinct increase in tenascin staining was noted; enhanced tenascin often paralleled increasing hyperplasia and dysplasia. In most cervical and vulvar carcinomas in situ, the reactions were intense and extended deeply and raggedly into the underlying stroma. Tenascin was selectively enhanced in the endocervical stroma around inflammed or metaplastic glands. The authors conclude that tenascin is increased in HPV infection associated with epithelial proliferation. Enhancement was most consistently strong and extensive in in situ carcinomas, suggesting a correlation with active phases of epithelial growth and stromal remodeling.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aufderheide E., Chiquet-Ehrismann R., Ekblom P. Epithelial-mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchyme. J Cell Biol. 1987 Jul;105(1):599–608. doi: 10.1083/jcb.105.1.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufderheide E., Ekblom P. Tenascin during gut development: appearance in the mesenchyme, shift in molecular forms, and dependence on epithelial-mesenchymal interactions. J Cell Biol. 1988 Dec;107(6 Pt 1):2341–2349. doi: 10.1083/jcb.107.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A. Participation of tenascin and transforming growth factor-beta in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res. 1989 Aug 1;49(15):4322–4325. [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. What distinguishes tenascin from fibronectin? FASEB J. 1990 Jun;4(9):2598–2604. doi: 10.1096/fasebj.4.9.1693347. [DOI] [PubMed] [Google Scholar]
- Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 1989 Aug;3(10):2141–2150. doi: 10.1096/fasebj.3.10.2666230. [DOI] [PubMed] [Google Scholar]
- Engel J. EGF-like domains in extracellular matrix proteins: localized signals for growth and differentiation? FEBS Lett. 1989 Jul 17;251(1-2):1–7. doi: 10.1016/0014-5793(89)81417-6. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Koukoulis G. K., Virtanen I. Extracellular matrix proteins and their receptors in the normal, hyperplastic and neoplastic breast. Cell Differ Dev. 1990 Dec 2;32(3):409–416. doi: 10.1016/0922-3371(90)90057-4. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Martinez-Lacabe V., Virtanen I., Sahlin K. M., Schwartz M. M. Differential distribution of tenascin and cellular fibronectins in acute and chronic renal allograft rejection. Lab Invest. 1992 Jul;67(1):71–79. [PubMed] [Google Scholar]
- Howeedy A. A., Virtanen I., Laitinen L., Gould N. S., Koukoulis G. K., Gould V. E. Differential distribution of tenascin in the normal, hyperplastic, and neoplastic breast. Lab Invest. 1990 Dec;63(6):798–806. [PubMed] [Google Scholar]
- Koch M., Wehrle-Haller B., Baumgartner S., Spring J., Brubacher D., Chiquet M. Epithelial synthesis of tenascin at tips of growing bronchi and graded accumulation in basement membrane and mesenchyme. Exp Cell Res. 1991 Jun;194(2):297–300. doi: 10.1016/0014-4827(91)90368-5. [DOI] [PubMed] [Google Scholar]
- Koukoulis G. K., Gould V. E., Bhattacharyya A., Gould J. E., Howeedy A. A., Virtanen I. Tenascin in normal, reactive, hyperplastic, and neoplastic tissues: biologic and pathologic implications. Hum Pathol. 1991 Jul;22(7):636–643. doi: 10.1016/0046-8177(91)90285-w. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liesi P., Dahl D., Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983 Mar;96(3):920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner V. A., Gumkowski F., Bigner D. D., Erickson H. P. Tenascin/hexabrachion in human skin: biochemical identification and localization by light and electron microscopy. J Cell Biol. 1989 Jun;108(6):2483–2493. doi: 10.1083/jcb.108.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Chiquet-Ehrismann R., Pearson C. A., Inaguma Y., Taya K., Kawarada Y., Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4621–4625. doi: 10.1073/pnas.84.13.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Halfter W., Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988 Dec;107(6 Pt 2):2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Bigotti A., Botti C., Castellani P., Risso A. M., Zardi L. Comparative analysis of the expression of the extracellular matrix protein tenascin in normal human fetal, adult and tumor tissues. Int J Cancer. 1991 Apr 1;47(6):811–816. doi: 10.1002/ijc.2910470603. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Zardi L. Tenascin: a hexameric adhesive glycoprotein. Int J Cancer Suppl. 1989;4:66–68. doi: 10.1002/ijc.2910440718. [DOI] [PubMed] [Google Scholar]
- Pearson C. A., Pearson D., Shibahara S., Hofsteenge J., Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-beta. EMBO J. 1988 Oct;7(10):2977–2982. doi: 10.1002/j.1460-2075.1988.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto A. L., Jones F. S., Cunningham B. A., Crossin K. L., Edelman G. M. Localization during development of alternatively spliced forms of cytotactin mRNA by in situ hybridization. J Cell Biol. 1990 Aug;111(2):685–698. doi: 10.1083/jcb.111.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjänen K. J. Epidemiology of human papillomavirus (HPV) infections and their associations with genital squamous cell cancer. Review article. APMIS. 1989 Nov;97(11):957–970. doi: 10.1111/j.1699-0463.1989.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Tervo K., Tervo T., van Setten G. B., Tarkkanen A., Virtanen I. Demonstration of tenascin-like immunoreactivity in rabbit corneal wounds. Acta Ophthalmol (Copenh) 1989 Jun;67(3):347–350. doi: 10.1111/j.1755-3768.1989.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Thesleff I., Mackie E., Vainio S., Chiquet-Ehrismann R. Changes in the distribution of tenascin during tooth development. Development. 1987 Oct;101(2):289–296. doi: 10.1242/dev.101.2.289. [DOI] [PubMed] [Google Scholar]
- Vollmer G., Siegal G. P., Chiquet-Ehrismann R., Lightner V. A., Arnholdt H., Knuppen R. Tenascin expression in the human endometrium and in endometrial adenocarcinomas. Lab Invest. 1990 Jun;62(6):725–730. [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989 Sep 1;49(17):4677–4681. [PubMed] [Google Scholar]