Abstract
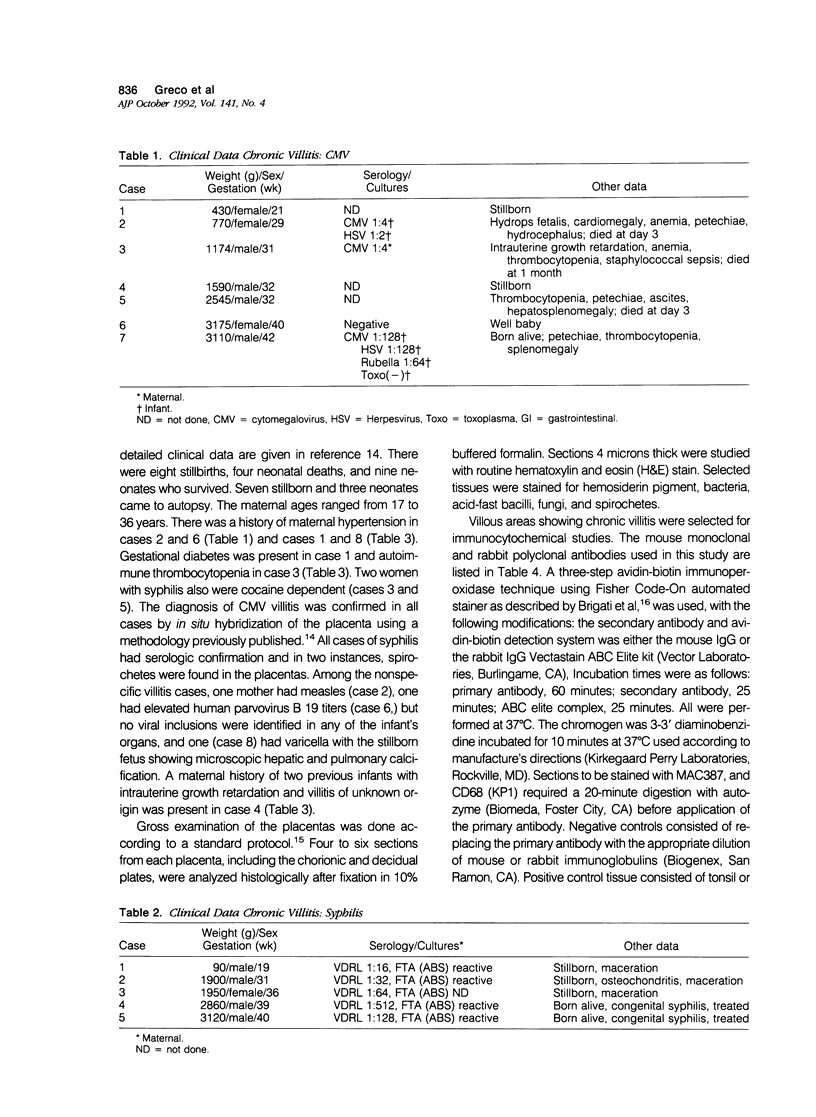
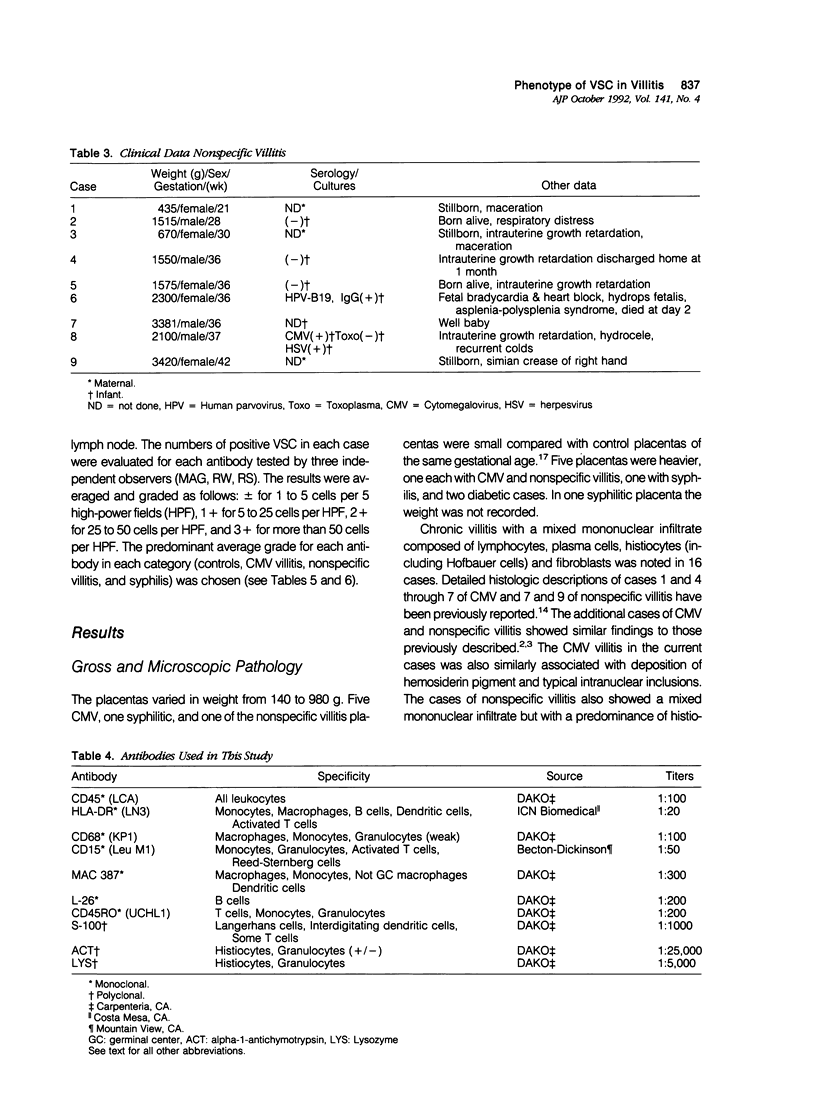
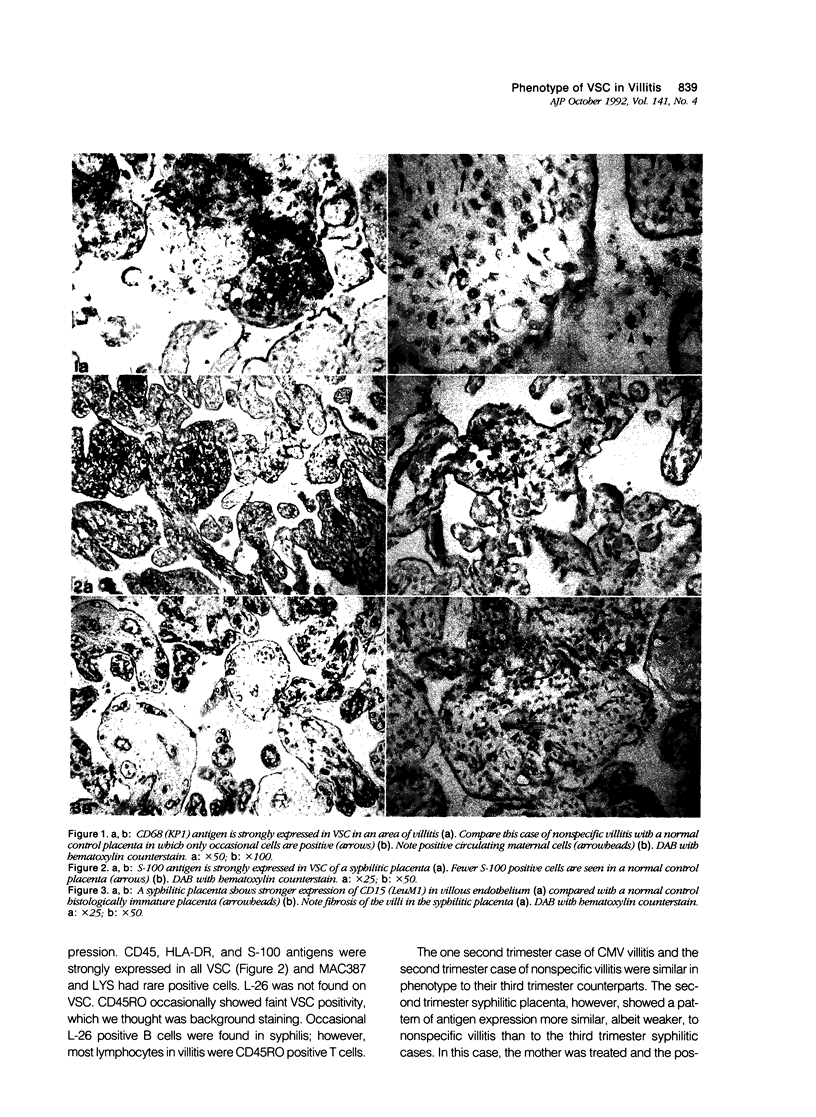
Villous stromal cells (VSC) play an important role in fetomaternal placental immune function. We studied the phenotype of VSC in infection by cytomegalovirus (CMV) and syphilis as well as nonspecific villitis and compared the findings with gestational age-matched controls. Monoclonal antibodies directed against total leukocytes, T cells, B cells, macrophages, dendritic cells, granulocytes and HLA-DR as well as polyclonal antibodies against S-100, alpha-1 antichymotrypsin, and lysozyme were used. In controls, the immunocytochemical response for each marker was either negative or weakly positive. In contrast, the VSC in CMV-infected and nonspecific villitis showed intense reactivity to various macrophage markers. In syphilis, reactivity with macrophage markers such as lysozyme and MAC387 were weaker, and reactivity to HLA-DR and S-100 was much stronger. Endothelial cells strongly expressed the monocyte/granulocyte marker CD15 in the diseased states, especially in syphilis, relative to controls. We conclude that the phenotype of VSC is altered in disease states and that the changes are dependent to some degree on the specific subset of chronic villitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Altshuler G., Russell P. The human placental villitides: a review of chronic intrauterine infection. Curr Top Pathol. 1975;60:64–112. [PubMed] [Google Scholar]
- Barthélémy H., Claudy A. Demonstration of T6+ and DR+ cells in human placenta. Arch Dermatol Res. 1987;279(7):495–497. doi: 10.1007/BF00412600. [DOI] [PubMed] [Google Scholar]
- Braunhut S. J., Blanc W. A., Ramanarayanan M., Marboe C., Mesa-Tejada R. Immunocytochemical localization of lysozyme and alpha-1-antichymotrypsin in the term human placenta: an attempt to characterize the Hofbauer cell. J Histochem Cytochem. 1984 Nov;32(11):1204–1210. doi: 10.1177/32.11.6548486. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Morrison L., Smith J. C. Expression of class II MHC gene products by macrophages in human uteroplacental tissue. Immunology. 1988 Apr;63(4):707–714. [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S. American Association of Pathologists president's address. New roles for the endothelium in inflammation and immunity. Am J Pathol. 1987 Dec;129(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Enders A. C., King B. F. The cytology of Hofbauer cells. Anat Rec. 1970 Jun;167(2):231–236. doi: 10.1002/ar.1091670211. [DOI] [PubMed] [Google Scholar]
- Fox H. The incidence and significance of Hofbauer cells in the mature human placenta. J Pathol Bacteriol. 1967 Apr;93(2):710–717. doi: 10.1002/path.1700930239. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Braverman M., Salafia C., Buckley P. The phenotype of human placental macrophages and its variation with gestational age. Am J Pathol. 1988 Dec;133(3):648–659. [PMC free article] [PubMed] [Google Scholar]
- Greco M. A., Kamat B. R., Demopoulos R. I. Placental protein distribution in maternal diabetes mellitus: an immunocytochemical study. Pediatr Pathol. 1989;9(6):679–690. doi: 10.3109/15513818909022375. [DOI] [PubMed] [Google Scholar]
- Labarrere C. A., Faulk W. P., McIntyre J. A. Villitis in normal term human placentae: frequency of the lesion determined by monoclonal antibody to HLA-DR antigen. J Reprod Immunol. 1989 Nov;16(2):127–135. doi: 10.1016/0165-0378(89)90022-3. [DOI] [PubMed] [Google Scholar]
- Labarrere C. A., McIntyre J. A., Faulk W. P. Immunohistologic evidence that villitis in human normal term placentas is an immunologic lesion. Am J Obstet Gynecol. 1990 Feb;162(2):515–522. doi: 10.1016/0002-9378(90)90421-3. [DOI] [PubMed] [Google Scholar]
- Labarrere C., Althabe O. Chronic villitis of unknown aetiology in recurrent intrauterine fetal growth retardation. Placenta. 1987 Mar-Apr;8(2):167–173. doi: 10.1016/0143-4004(87)90019-1. [DOI] [PubMed] [Google Scholar]
- Labarrere C., Althabe O., Telenta M. Chronic villitis of unknown aetiology in placentae of idiopathic small for gestational age infants. Placenta. 1982 Jul-Sep;3(3):309–317. doi: 10.1016/s0143-4004(82)80007-6. [DOI] [PubMed] [Google Scholar]
- Labarrere C., Mullen E. Fibrinoid and trophoblastic necrosis with massive chronic intervillositis: an extreme variant of villitis of unknown etiology. Am J Reprod Immunol Microbiol. 1987 Nov;15(3):85–91. doi: 10.1111/j.1600-0897.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Lessin D. L., Hunt J. S., King C. R., Wood G. W. Antigen expression by cells near the maternal-fetal interface. Am J Reprod Immunol Microbiol. 1988 Jan;16(1):1–7. doi: 10.1111/j.1600-0897.1988.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Mues B., Langer D., Zwadlo G., Sorg C. Phenotypic characterization of macrophages in human term placenta. Immunology. 1989 Jul;67(3):303–307. [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Recruitment of neutrophils in the local endotoxin response: association with de novo endothelial expression of endothelial leukocyte adhesion molecule-1. Lab Invest. 1991 Feb;64(2):295–299. [PubMed] [Google Scholar]
- Nakamura Y., Ohta Y. Immunohistochemical study of human placental stromal cells. Hum Pathol. 1990 Sep;21(9):936–940. doi: 10.1016/0046-8177(90)90178-8. [DOI] [PubMed] [Google Scholar]
- Redline R. W., Abramowsky C. R. Clinical and pathologic aspects of recurrent placental villitis. Hum Pathol. 1985 Jul;16(7):727–731. doi: 10.1016/s0046-8177(85)80159-3. [DOI] [PubMed] [Google Scholar]
- Russell P., Atkinson K., Krishnan L. Recurrent reproductive failure due to severe placental villitis of unknown etiology. J Reprod Med. 1980 Feb;24(2):93–98. [PubMed] [Google Scholar]
- Russell P. Inflammatory lesions of the human placenta. III. The histopathology of villitis of unknown aetiology. Placenta. 1980 Jul-Sep;1(3):227–244. doi: 10.1016/s0143-4004(80)80005-1. [DOI] [PubMed] [Google Scholar]
- Sachdev R., Nuovo G. J., Kaplan C., Greco M. A. In situ hybridization analysis for cytomegalovirus in chronic villitis. Pediatr Pathol. 1990;10(6):909–917. doi: 10.3109/15513819009064726. [DOI] [PubMed] [Google Scholar]
- Salafia C. M., Haynes N., Merluzzi V. J., Rothlein R. Distribution of ICAM-1 within decidua and placenta and its gestational age-associated changes. Pediatr Pathol. 1991 May-Jun;11(3):381–388. doi: 10.3109/15513819109064774. [DOI] [PubMed] [Google Scholar]
- Schwartz D. A., Khan R., Stoll B. Characterization of the fetal inflammatory response to cytomegalovirus placentitis. An immunohistochemical study. Arch Pathol Lab Med. 1992 Jan;116(1):21–27. [PubMed] [Google Scholar]
- Singer D. B. The placenta in pregnancies complicated by diabetes mellitus. Perspect Pediatr Pathol. 1984 Fall;8(3):199–212. [PubMed] [Google Scholar]
- Sutton L., Gadd M., Mason D. Y., Redman C. W. Cells bearing class II MHC antigens in the human placenta and amniochorion. Immunology. 1986 May;58(1):23–29. [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Wood G. W. Mononuclear phagocytes in the human placenta. Placenta. 1980 Apr-Jun;1(2):113–123. doi: 10.1016/s0143-4004(80)80019-1. [DOI] [PubMed] [Google Scholar]



