Abstract
Thrombospondin is part of a family of adhesive glycoproteins and is involved in a number of physiologic processes such as angiogenesis and neurite outgrowth. Immunohistochemical localization of thrombospondin in normal human brains was investigated in the hippocampus and inferior temporal cortex. Two antibodies (one polyclonal and one monoclonal) against thrombospondin-labeled microvessels, glial cells, and a subpopulation of pyramidal neurons. The distribution of thrombospondin staining in patients with Alzheimer's disease was found to be comparable to control subjects. However, in patients with Alzheimer's disease a subset of pyramidal neurons that may be vulnerable in Alzheimer's disease exhibited decreased staining. This decrease in the intensity of labeling might constitute a marker for a neuronal population prone to early degeneration. In addition, thrombospondin staining was demonstrated in senile plaques in Alzheimer's disease. These results suggest that thrombospondin may be involved in the process of neuronal degeneration and senile plaque formation.
Full text
PDF
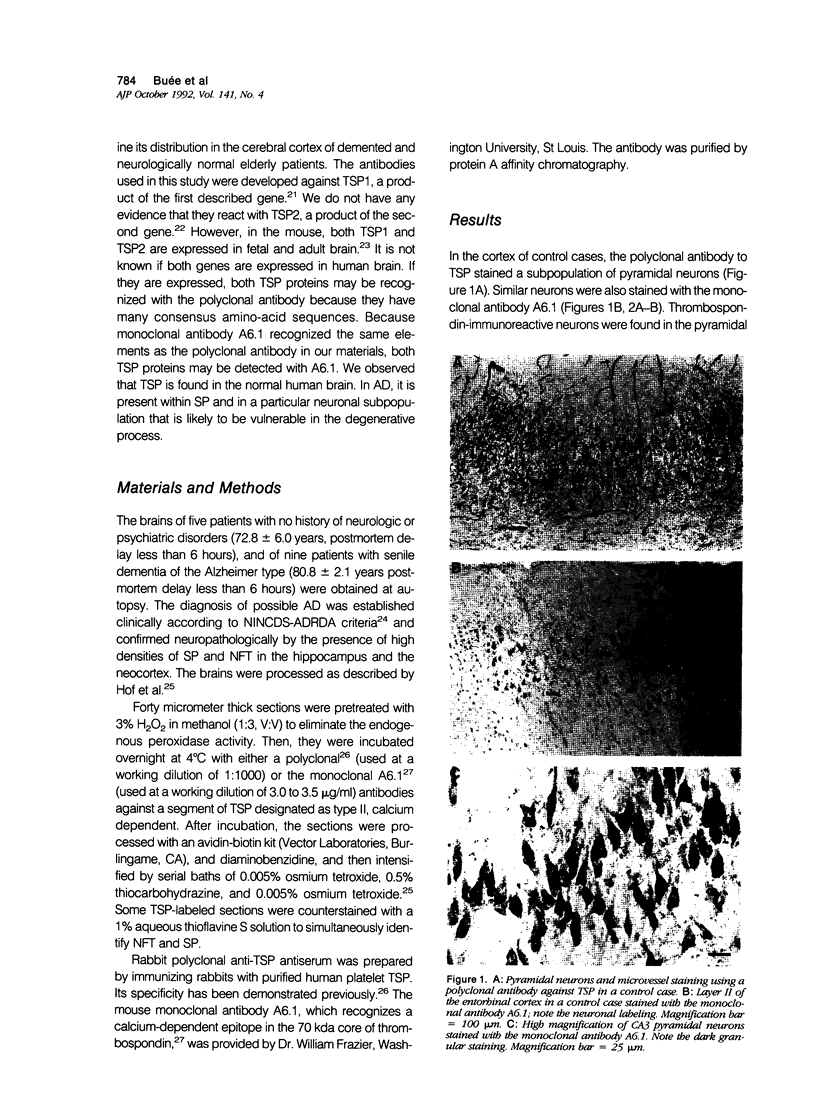




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Asch A. S., Leung L. L., Shapiro J., Nachman R. L. Human brain glial cells synthesize thrombospondin. Proc Natl Acad Sci U S A. 1986 May;83(9):2904–2908. doi: 10.1073/pnas.83.9.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Devarayalu S., Li P., Disteche C. M., Framson P. A second thrombospondin gene in the mouse is similar in organization to thrombospondin 1 but does not respond to serum. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8636–8640. doi: 10.1073/pnas.88.19.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacourte A., Flament S., Dibe E. M., Hublau P., Sablonnière B., Hémon B., Shérrer V., Défossez A. Pathological proteins Tau 64 and 69 are specifically expressed in the somatodendritic domain of the degenerating cortical neurons during Alzheimer's disease. Demonstration with a panel of antibodies against Tau proteins. Acta Neuropathol. 1990;80(2):111–117. doi: 10.1007/BF00308912. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Galvin N. J., O'Rourke K. M., Frazier W. A. Monoclonal antibodies that recognize calcium-dependent structures of human thrombospondin. Characterization and mapping of their epitopes. J Biol Chem. 1986 Feb 5;261(4):1962–1968. [PubMed] [Google Scholar]
- Fischer V. W., Siddiqi A., Yusufaly Y. Altered angioarchitecture in selected areas of brains with Alzheimer's disease. Acta Neuropathol. 1990;79(6):672–679. doi: 10.1007/BF00294246. [DOI] [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondins. Curr Opin Cell Biol. 1991 Oct;3(5):792–799. doi: 10.1016/0955-0674(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof P. R., Cox K., Morrison J. H. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol. 1990 Nov 1;301(1):44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Kromer L. J., Damasio A. R. Perforant pathway changes and the memory impairment of Alzheimer's disease. Ann Neurol. 1986 Oct;20(4):472–481. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- Kisilevsky R. Heparan sulfate proteoglycans in amyloidogenesis: an epiphenomenon, a unique factor, or the tip of a more fundamental process? Lab Invest. 1990 Nov;63(5):589–591. [PubMed] [Google Scholar]
- Laherty C. D., O'Rourke K., Wolf F. W., Katz R., Seldin M. F., Dixit V. M. Characterization of mouse thrombospondin 2 sequence and expression during cell growth and development. J Biol Chem. 1992 Feb 15;267(5):3274–3281. [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Marcyniuk B. Some morphometric observations on the cerebral cortex and hippocampus in presenile Alzheimer's disease, senile dementia of Alzheimer type and Down's syndrome in middle age. J Neurol Sci. 1985 Jul;69(3):139–159. doi: 10.1016/0022-510x(85)90129-7. [DOI] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Hanning R., Mosher D. F. Binding and degradation of platelet thrombospondin by cultured fibroblasts. J Cell Biol. 1984 Jan;98(1):22–28. doi: 10.1083/jcb.98.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Physiology of thrombospondin. Annu Rev Med. 1990;41:85–97. doi: 10.1146/annurev.me.41.020190.000505. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer K. M., Emmett C. J., Venstrom K. A., Reichardt L. F. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991 Mar;6(3):345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- O'Shea K. S., Dixit V. M. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988 Dec;107(6 Pt 2):2737–2748. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea K. S., Liu L. H., Dixit V. M. Thrombospondin and a 140 kd fragment promote adhesion and neurite outgrowth from embryonic central and peripheral neurons and from PC12 cells. Neuron. 1991 Aug;7(2):231–237. doi: 10.1016/0896-6273(91)90261-w. [DOI] [PubMed] [Google Scholar]
- O'Shea K. S., Rheinheimer J. S., Dixit V. M. Deposition and role of thrombospondin in the histogenesis of the cerebellar cortex. J Cell Biol. 1990 Apr;110(4):1275–1283. doi: 10.1083/jcb.110.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter L. S., Barrón E., Saperia D., Chui H. C. Association between vascular basement membrane components and the lesions of Alzheimer's disease. J Neurosci Res. 1991 Dec;30(4):673–681. doi: 10.1002/jnr.490300411. [DOI] [PubMed] [Google Scholar]
- Roberts D. D. Interactions of thrombospondin with sulfated glycolipids and proteoglycans of human melanoma cells. Cancer Res. 1988 Dec 1;48(23):6785–6793. [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood J. A., Roberts D. D., Spitalnik S. L., Marsh K., Harvey E. B., Miller L. H., Howard R. J. Studies of the receptors on melanoma cells for Plasmodium falciparum infected erythrocytes. Am J Trop Med Hyg. 1989 Feb;40(2):119–127. doi: 10.4269/ajtmh.1989.40.119. [DOI] [PubMed] [Google Scholar]
- Taraboletti G., Roberts D. D., Liotta L. A. Thrombospondin-induced tumor cell migration: haptotaxis and chemotaxis are mediated by different molecular domains. J Cell Biol. 1987 Nov;105(5):2409–2415. doi: 10.1083/jcb.105.5.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G., Roberts D., Liotta L. A., Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990 Aug;111(2):765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., Hansen L. A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991 Oct;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Peck A., DeTeresa R., Schechter R., Horoupian D. S. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981 Aug;10(2):184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]






