Abstract
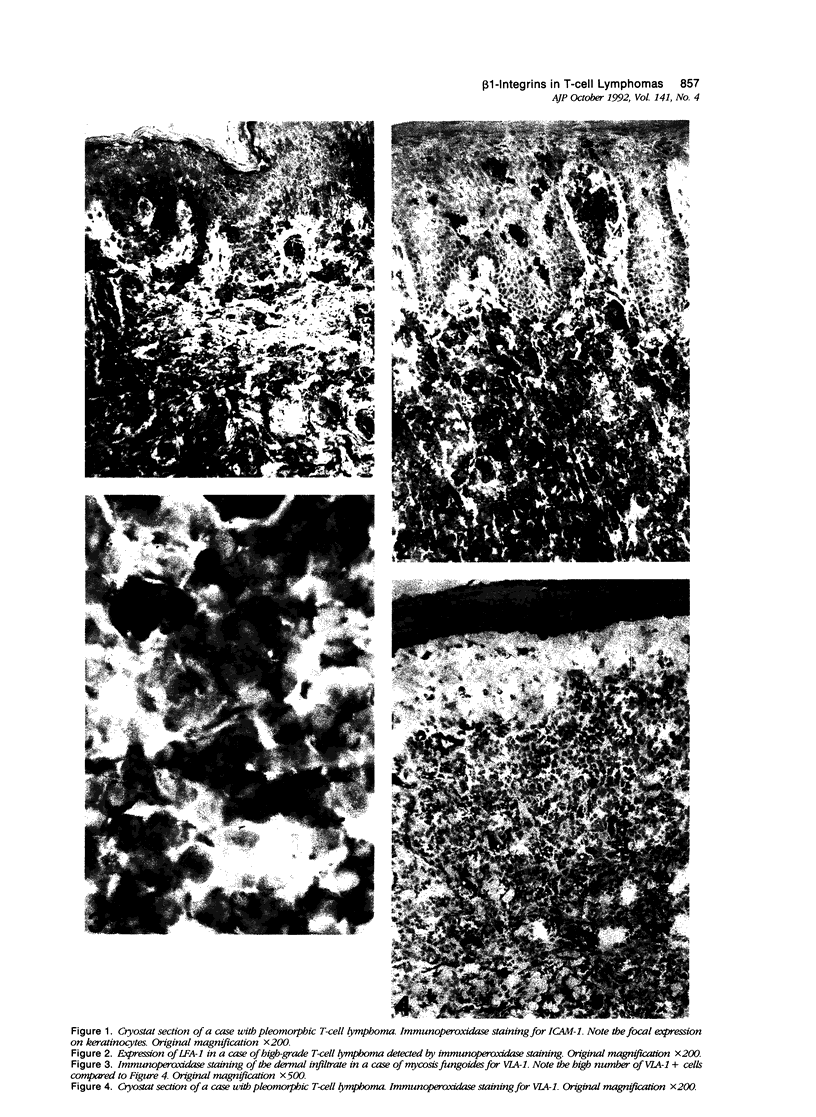
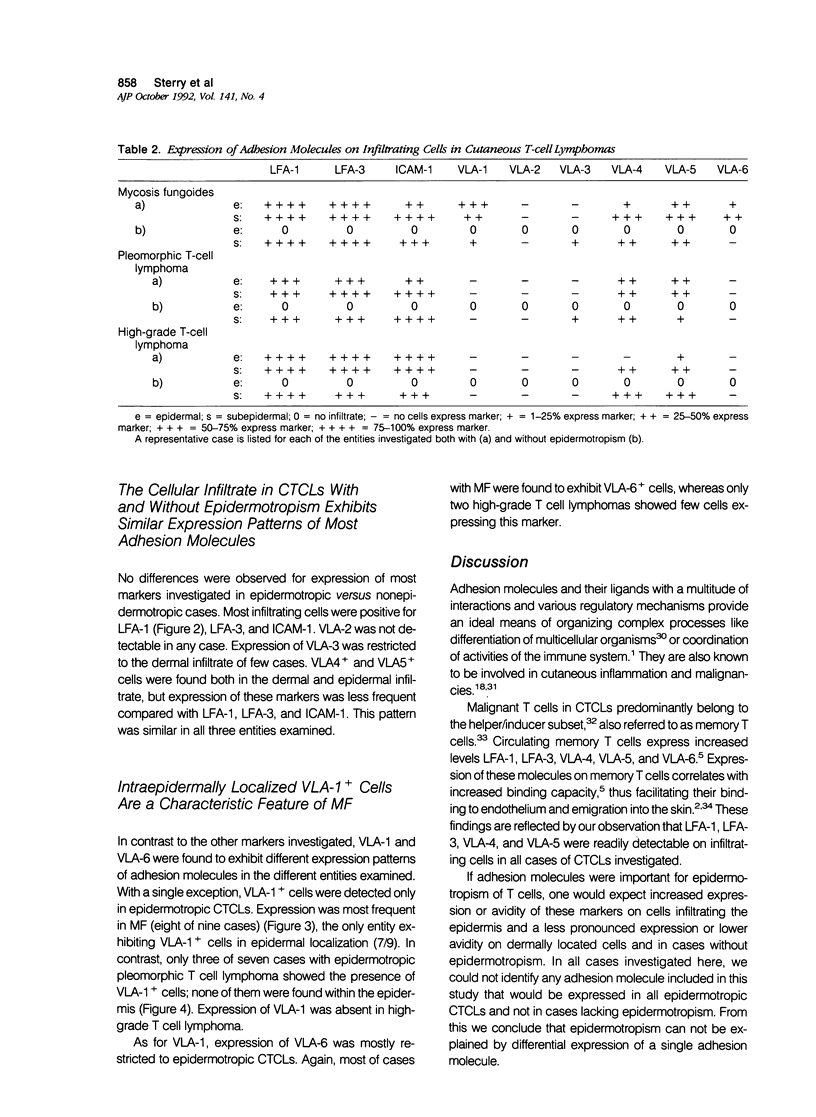
To better understand the molecular mechanisms of epidermotropism, we immunohistochemically analyzed the expression pattern of adhesion molecules belonging to the integrin and immunoglobulin superfamilies in cases of mycosis fungoides (MF) (n = 15), pleomorphic T cell lymphoma (n = 10), and high-grade T cell lymphoma (n = 7). The cutaneous T cell lymphomas (CTCLs) investigated were categorized into cases with or without epidermotropism. Focal neoexpression of ICAM-1 on keratinocytes was restricted to epidermotropic lymphomas. Both LFA-1 and LFA-3 were expressed on infiltrating cells in all cases investigated. In contrast, beta 1-integrins showed differential expression, most prominent in the case of VLA-1 and VLA-6: These molecules were present on infiltrating cells in most cases with epidermotropic MF and absent in most other CTCLs. We conclude that the phenomenon of epidermotropism might involve different sets of adhesion molecules in different entities of CTCL, with VLA-1 being the most influential beta 1-integrin in the case of MF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger C. L., Warburton D., Raafat J., LoGerfo P., Edelson R. L. Cutaneous T-cell lymphoma: neoplasm of T cells with helper activity. Blood. 1979 Apr;53(4):642–651. [PubMed] [Google Scholar]
- Boehncke W. H., Krettek S., Parwaresch M. R., Sterry W. Demonstration of clonal disease in early mycosis fungoides. Am J Dermatopathol. 1992 Apr;14(2):95–99. doi: 10.1097/00000372-199204000-00002. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Hemler M. E., Crouse C., Takada Y., Sonnenberg A. Multiple very late antigen (VLA) heterodimers on platelets. Evidence for distinct VLA-2, VLA-5 (fibronectin receptor), and VLA-6 structures. J Biol Chem. 1988 Jun 5;263(16):7660–7665. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hemler M. E., Jacobson J. G., Strominger J. L. Biochemical characterization of VLA-1 and VLA-2. Cell surface heterodimers on activated T cells. J Biol Chem. 1985 Dec 5;260(28):15246–15252. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kaufmann R., Frösch D., Westphal C., Weber L., Klein C. E. Integrin VLA-3: ultrastructural localization at cell-cell contact sites of human cell cultures. J Cell Biol. 1989 Oct;109(4 Pt 1):1807–1815. doi: 10.1083/jcb.109.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Kyan-Aung U., Haskard D. O., Poston R. N., Thornhill M. H., Lee T. H. Endothelial leukocyte adhesion molecule-1 and intercellular adhesion molecule-1 mediate the adhesion of eosinophils to endothelial cells in vitro and are expressed by endothelium in allergic cutaneous inflammation in vivo. J Immunol. 1991 Jan 15;146(2):521–528. [PubMed] [Google Scholar]
- Mackay C. R. Lymphocyte homing. Skin-seeking memory T cells. Nature. 1991 Feb 28;349(6312):737–738. doi: 10.1038/349737a0. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Medeiros L. J., Weiss L. M., Picker L. J., Clayberger C., Horning S. J., Krensky A. M., Warnke R. A. Expression of LFA-1 in non-Hodgkin's lymphoma. Cancer. 1989 Jan 15;63(2):255–259. doi: 10.1002/1097-0142(19890115)63:2<255::aid-cncr2820630209>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Griffiths C. E., Baadsgaard O., Voorhees J. J., Hanson C. A., Cooper K. D. Markedly diminished epidermal keratinocyte expression of intercellular adhesion molecule-1 (ICAM-1) in Sézary syndrome. JAMA. 1989 Apr 21;261(15):2217–2221. [PubMed] [Google Scholar]
- Nickoloff B. J. Role of interferon-gamma in cutaneous trafficking of lymphocytes with emphasis on molecular and cellular adhesion events. Arch Dermatol. 1988 Dec;124(12):1835–1843. [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Shiohara T., Moriya N., Mochizuki T., Nagashima M. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987 Jul;89(1):8–14. [PubMed] [Google Scholar]
- Slater D. N. Cutaneous lymphoproliferative disorders: an assessment of recent investigative techniques. Br J Dermatol. 1991 Apr;124(4):309–323. doi: 10.1111/j.1365-2133.1991.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Springer T. A. The sensation and regulation of interactions with the extracellular environment: the cell biology of lymphocyte adhesion receptors. Annu Rev Cell Biol. 1990;6:359–402. doi: 10.1146/annurev.cb.06.110190.002043. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Sterry W., Bruhn S., Künne N., Lichtenberg B., Weber-Matthiesen K., Brasch J., Mielke V. Dominance of memory over naive T cells in contact dermatitis is due to differential tissue immigration. Br J Dermatol. 1990 Jul;123(1):59–64. doi: 10.1111/j.1365-2133.1990.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Sterry W. Mycosis fungoides. Curr Top Pathol. 1985;74:167–223. doi: 10.1007/978-3-642-69574-2_5. [DOI] [PubMed] [Google Scholar]
- Wallner B. P., Frey A. Z., Tizard R., Mattaliano R. J., Hession C., Sanders M. E., Dustin M. L., Springer T. A. Primary structure of lymphocyte function-associated antigen 3 (LFA-3). The ligand of the T lymphocyte CD2 glycoprotein. J Exp Med. 1987 Oct 1;166(4):923–932. doi: 10.1084/jem.166.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]






