Abstract
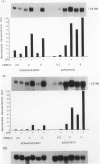
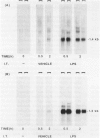
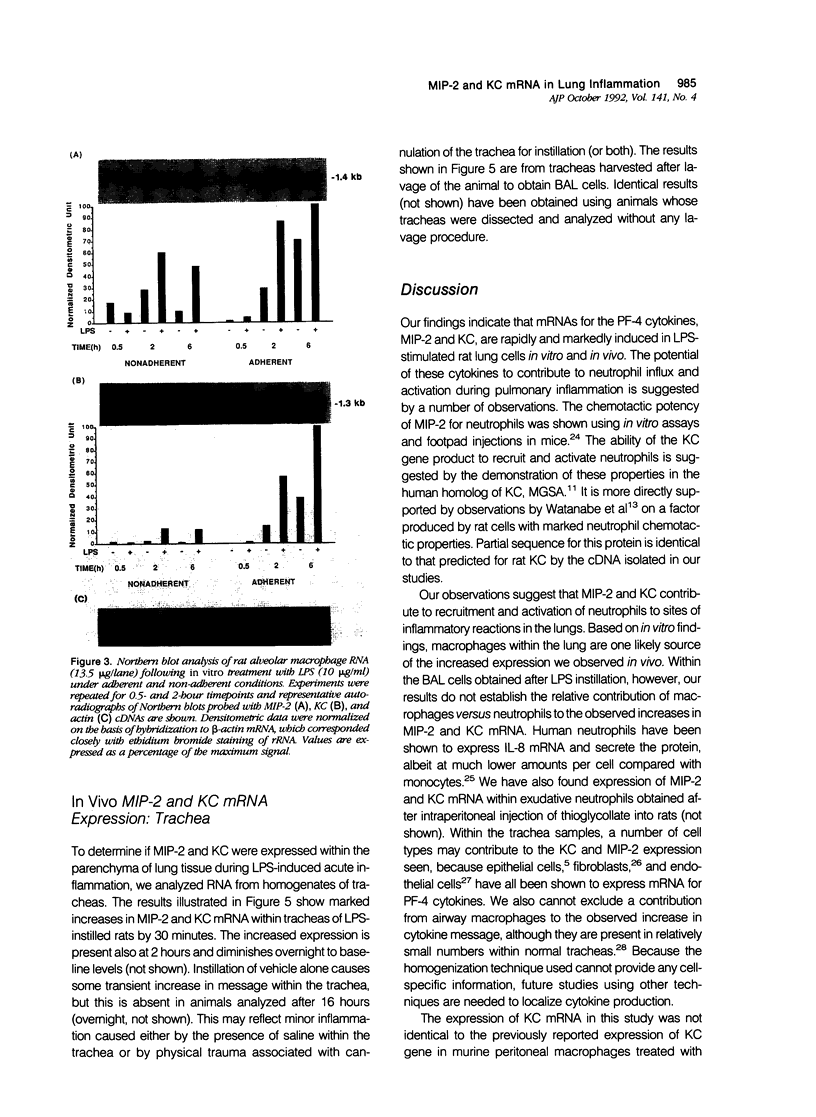
This study sought to test the hypothesis that expression of mRNA for two cytokines, macrophage inflammatory protein-2 (MIP-2) and the KC gene product, is induced in rat lung cells during inflammatory responses in vitro and in vivo. Macrophage inflammatory protein-2 and KC are members of the platelet-factor 4 (PF-4) cytokine superfamily that cause marked neutrophil chemotaxis and activation in vitro. To investigate expression of the genes for MIP-2 and KC in rat models of lung injury, cDNA probes for these cytokines in the rat were made from polymerase chain reaction (PCR) products generated using mouse sequence-derived primers. Sequence analysis of these cDNAs showed marked homology to known murine sequences (89% and 92% MIP-2 and KC, respectively). These cDNAs were first used to study the expression of these two genes in rat alveolar macrophages (AMs) in vitro by Northern blot hybridization. Lipopolysaccharide (LPS) treatment of rat AMs in vitro caused marked increases in mRNA for both KC and MIP-2 within 30 minutes, which persisted through the 6 hours measured. To study expression during inflammation in vivo, rats were treated with LPS by intratracheal instillation. Bronchoalveolar lavage (BAL) cells and whole trachea homogenates were analyzed. There was a marked and rapid increase in MIP-2 and KC mRNA levels within both BAL cells and trachea homogenates after LPS instillation. The results support the hypothesis that MIP-2 and KC cytokines contribute to neutrophil chemotaxis and activation in this rat model of acute pulmonary inflammation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anisowicz A., Zajchowski D., Stenman G., Sager R. Functional diversity of gro gene expression in human fibroblasts and mammary epithelial cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9645–9649. doi: 10.1073/pnas.85.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni F., Cassatella M. A., Rossi F., Ceska M., Dewald B., Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991 Mar 1;173(3):771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Eierman D. F., Johnson C. E., Haskill J. S. Human monocyte inflammatory mediator gene expression is selectively regulated by adherence substrates. J Immunol. 1989 Mar 15;142(6):1970–1976. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Helinski E. H., Bielat K. L., Ovak G. M., Pauly J. L. Long-term cultivation of functional human macrophages in Teflon dishes with serum-free media. J Leukoc Biol. 1988 Aug;44(2):111–121. doi: 10.1002/jlb.44.2.111. [DOI] [PubMed] [Google Scholar]
- Introna M., Bast R. C., Jr, Tannenbaum C. S., Hamilton T. A., Adams D. O. The effect of LPS on expression of the early "competence" genes JE and KC in murine peritoneal macrophages. J Immunol. 1987 Jun 1;138(11):3891–3896. [PubMed] [Google Scholar]
- Kasahara K., Strieter R. M., Chensue S. W., Standiford T. J., Kunkel S. L. Mononuclear cell adherence induces neutrophil chemotactic factor/interleukin-8 gene expression. J Leukoc Biol. 1991 Sep;50(3):287–295. doi: 10.1002/jlb.50.3.287. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Standiford T., Kasahara K., Strieter R. M. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp Lung Res. 1991 Jan-Feb;17(1):17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- Lehnert B. E., Valdez Y. E., Sebring R. J., Lehnert N. M., Saunders G. C., Steinkamp J. A. Airway intra-luminal macrophages: evidence of origin and comparisons to alveolar macrophages. Am J Respir Cell Mol Biol. 1990 Oct;3(4):377–391. doi: 10.1165/ajrcmb/3.4.377. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]). Am J Respir Cell Mol Biol. 1990 Jun;2(6):479–486. doi: 10.1165/ajrcmb/2.6.479. [DOI] [PubMed] [Google Scholar]
- Moser B., Clark-Lewis I., Zwahlen R., Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990 May 1;171(5):1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo P., Alberta J., Wen D. Z., Graycar J. L., Derynck R., Stiles C. D. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J Biol Chem. 1989 Mar 5;264(7):4133–4137. [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Ribeiro R. A., Flores C. A., Cunha F. Q., Ferreira S. H. IL-8 causes in vivo neutrophil migration by a cell-dependent mechanism. Immunology. 1991 Aug;73(4):472–477. [PMC free article] [PubMed] [Google Scholar]
- Rot A. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 1991 Jan;3(1):21–27. doi: 10.1016/1043-4666(91)90006-y. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Chensue S. W., Basha M. A., Standiford T. J., Lynch J. P., Baggiolini M., Kunkel S. L. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990 Apr;2(4):321–326. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C. S., Major J. A., Poptic E. J., DiCorleto P. E., Hamilton T. A. Lipopolysaccharide induces competence genes JE and KC in Balb/C 3T3 cells. J Cell Physiol. 1990 Jul;144(1):77–83. doi: 10.1002/jcp.1041440111. [DOI] [PubMed] [Google Scholar]
- Tekamp-Olson P., Gallegos C., Bauer D., McClain J., Sherry B., Fabre M., van Deventer S., Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990 Sep 1;172(3):911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Watson L. R., Yin S. M., Guo K. Z., Wang P., Thang H., del Castillo J. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am J Pathol. 1991 Jun;138(6):1485–1496. [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Konishi K., Fujioka M., Kinoshita S., Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989 Nov 25;264(33):19559–19563. [PubMed] [Google Scholar]
- Wen D. Z., Rowland A., Derynck R. Expression and secretion of gro/MGSA by stimulated human endothelial cells. EMBO J. 1989 Jun;8(6):1761–1766. doi: 10.1002/j.1460-2075.1989.tb03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Davatelis G., Sherry B., Beutler B., Hesse D. G., Nguyen H. T., Moldawer L. L., Nathan C. F., Lowry S. F., Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988 Feb 1;167(2):570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe S. D., Sherry B., Juers D., Davatelis G., Yurt R. W., Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989 Jan;86(2):612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]