Abstract
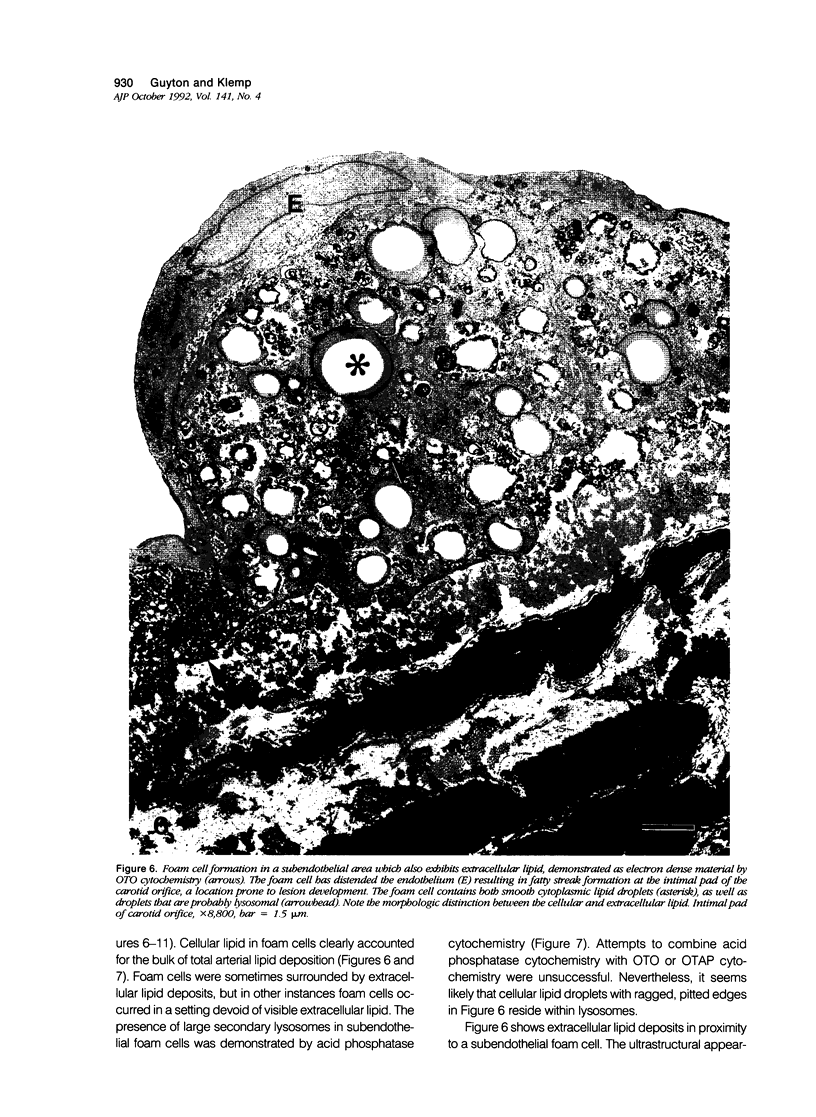
Subendothelial accumulation of extracellular liposomes rich in unesterified cholesterol has been described as an early feature of atherosclerosis induced by cholesterol feeding in rabbits. Beta-very-low-density lipoproteins, however, the presumed source of atherogenic lipid in this animal model, contain mostly esterified cholesterol. The purpose of this study was to test for the presence of extracellular neutral lipid deposits consistent with esterified cholesterol, by employing new electron microscopic techniques. Rabbits were fed 0.5% cholesterol, 5% butter for 0, 1, 2, and 4 weeks. The lipid-preserving ultrastructural techniques showed, in control and atherosclerotic rabbit arteries, neutral lipid droplets adherent to the endothelial luminal surface. After 1 to 2 weeks, subendothelial extracellular deposits of mostly membranous lipid appeared; these deposits contained variable amounts of neutral lipid. At the same time, cytoplasmic neutral lipid droplets appeared in smooth muscle cells and in a small number of subendothelial macrophagelike cells. After 4 weeks, monocytic infiltration and macrophage foam cell development were prominent, but abundant extracellular lipid deposits also were found. Therefore, in arteries of cholesterol-fed rabbits, deposition of membranous and neutral lipid in the extracellular space and neutral lipid accumulation in resident arterial cells are early and probably independent events, both occurring before monocytic infiltration of the arterial intima.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocan T. M., Guyton J. R. Human aortic fibrolipid lesions. Progenitor lesions for fibrous plaques, exhibiting early formation of the cholesterol-rich core. Am J Pathol. 1985 Aug;120(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Bocan T. M., Schifani T. A., Guyton J. R. Ultrastructure of the human aortic fibrolipid lesion. Formation of the atherosclerotic lipid-rich core. Am J Pathol. 1986 Jun;123(3):413–424. [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Camejo G. The interaction of lipids and lipoproteins with the intercellular matrix of arterial tissue: its possible role in atherogenesis. Adv Lipid Res. 1982;19:1–53. doi: 10.1016/b978-0-12-024919-0.50007-2. [DOI] [PubMed] [Google Scholar]
- Chao F. F., Amende L. M., Blanchette-Mackie E. J., Skarlatos S. I., Gamble W., Resau J. H., Mergner W. T., Kruth H. S. Unesterified cholesterol-rich lipid particles in atherosclerotic lesions of human and rabbit aortas. Am J Pathol. 1988 Apr;131(1):73–83. [PMC free article] [PubMed] [Google Scholar]
- Frank J. S., Fogelman A. M. Ultrastructure of the intima in WHHL and cholesterol-fed rabbit aortas prepared by ultra-rapid freezing and freeze-etching. J Lipid Res. 1989 Jul;30(7):967–978. [PubMed] [Google Scholar]
- Guyton J. R., Bocan T. M., Schifani T. A. Quantitative ultrastructural analysis of perifibrous lipid and its association with elastin in nonatherosclerotic human aorta. Arteriosclerosis. 1985 Nov-Dec;5(6):644–652. doi: 10.1161/01.atv.5.6.644. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Klemp K. F., Mims M. P. Altered ultrastructural morphology of self-aggregated low density lipoproteins: coalescence of lipid domains forming droplets and vesicles. J Lipid Res. 1991 Jun;32(6):953–962. [PubMed] [Google Scholar]
- Guyton J. R., Klemp K. F. Ultrastructural discrimination of lipid droplets and vesicles in atherosclerosis: value of osmium-thiocarbohydrazide-osmium and tannic acid-paraphenylenediamine techniques. J Histochem Cytochem. 1988 Oct;36(10):1319–1328. doi: 10.1177/36.10.2458408. [DOI] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C. Early atherogenesis in White Carneau pigeons. II. Ultrastructural and cytochemical observations. Am J Pathol. 1985 May;119(2):210–222. [PMC free article] [PubMed] [Google Scholar]
- Khoo J. C., Miller E., McLoughlin P., Steinberg D. Enhanced macrophage uptake of low density lipoprotein after self-aggregation. Arteriosclerosis. 1988 Jul-Aug;8(4):348–358. doi: 10.1161/01.atv.8.4.348. [DOI] [PubMed] [Google Scholar]
- Kramsch D. M., Hollander W. The interaction of serum and arterial lipoproteins with elastin of the arterial intima and its role in the lipid accumulation in atherosclerotic plaques. J Clin Invest. 1973 Feb;52(2):236–247. doi: 10.1172/JCI107180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruth H. S. Filipin-positive, oil red O-negative particles in atherosclerotic lesions induced by cholesterol feeding. Lab Invest. 1984 Jan;50(1):87–93. [PubMed] [Google Scholar]
- Kruth H. S., Fry D. L. Histochemical detection and differentiation of free and esterified cholesterol in swine atherosclerosis using filipin. Exp Mol Pathol. 1984 Jun;40(3):288–294. doi: 10.1016/0014-4800(84)90046-7. [DOI] [PubMed] [Google Scholar]
- Kruth H. S. Subendothelial accumulation of unesterified cholesterol. An early event in atherosclerotic lesion development. Atherosclerosis. 1985 Nov;57(2-3):337–341. doi: 10.1016/0021-9150(85)90045-0. [DOI] [PubMed] [Google Scholar]
- Mora R., Lupu F., Simionescu N. Cytochemical localization of beta-lipoproteins and their components in successive stages of hyperlipidemic atherogenesis of rabbit aorta. Atherosclerosis. 1989 Oct;79(2-3):183–195. doi: 10.1016/0021-9150(89)90123-8. [DOI] [PubMed] [Google Scholar]
- Mora R., Lupu F., Simionescu N. Prelesional events in atherogenesis. Colocalization of apolipoprotein B, unesterified cholesterol and extracellular phospholipid liposomes in the aorta of hyperlipidemic rabbit. Atherosclerosis. 1987 Oct;67(2-3):143–154. doi: 10.1016/0021-9150(87)90274-7. [DOI] [PubMed] [Google Scholar]
- Pitas R. E. Expression of the acetyl low density lipoprotein receptor by rabbit fibroblasts and smooth muscle cells. Up-regulation by phorbol esters. J Biol Chem. 1990 Jul 25;265(21):12722–12727. [PubMed] [Google Scholar]
- Podet E. J., Shaffer D. R., Gianturco S. H., Bradley W. A., Yang C. Y., Guyton J. R. Interaction of low density lipoproteins with human aortic elastin. Arterioscler Thromb. 1991 Jan-Feb;11(1):116–122. doi: 10.1161/01.atv.11.1.116. [DOI] [PubMed] [Google Scholar]
- Richardson M., Hatton M. W., Moore S. Proteoglycan distribution in the intima and media of the aortas of young and aging rabbits: an ultrastructural study. Atherosclerosis. 1988 Jun;71(2-3):243–256. doi: 10.1016/0021-9150(88)90149-9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Tsukada T., Gown A. M., Ross R. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987 Jan-Feb;7(1):9–23. doi: 10.1161/01.atv.7.1.9. [DOI] [PubMed] [Google Scholar]
- Sambandam T., Baker J. R., Christner J. E., Ekborg S. L. Specificity of the low density lipoprotein-glycosaminoglycan interaction. Arterioscler Thromb. 1991 May-Jun;11(3):561–568. doi: 10.1161/01.atv.11.3.561. [DOI] [PubMed] [Google Scholar]
- Schwenke D. C., Carew T. E. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. I. Focal increases in arterial LDL concentration precede development of fatty streak lesions. Arteriosclerosis. 1989 Nov-Dec;9(6):895–907. doi: 10.1161/01.atv.9.6.895. [DOI] [PubMed] [Google Scholar]
- Scow R. O., Blanchette-Mackie E. J., Smith L. C. Role of capillary endothelium in the clearance of chylomicrons. A model for lipid transport from blood by lateral diffusion in cell membranes. Circ Res. 1976 Aug;39(2):149–162. doi: 10.1161/01.res.39.2.149. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Mora R., Vasile E., Lupu F., Filip D. A., Simionescu M. Prelesional modifications of the vessel wall in hyperlipidemic atherogenesis. Extracellular accumulation of modified and reassembled lipoproteins. Ann N Y Acad Sci. 1990;598:1–16. doi: 10.1111/j.1749-6632.1990.tb42271.x. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Vasile E., Lupu F., Popescu G., Simionescu M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am J Pathol. 1986 Apr;123(1):109–125. [PMC free article] [PubMed] [Google Scholar]
- Smith E. B. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res. 1974;12(0):1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Srinivasan S. R., Vijayagopal P., Eberle K., Radhakrishnamurthy B., Berenson G. S. Low-density lipoprotein binding affinity of arterial wall proteoglycans: characteristics of a chondroitin sulfate proteoglycan subfraction. Biochim Biophys Acta. 1989 Nov 28;1006(2):159–166. doi: 10.1016/0005-2760(89)90190-2. [DOI] [PubMed] [Google Scholar]
- Wagner W. D., Edwards I. J., St Clair R. W., Barakat H. Low density lipoprotein interaction with artery derived proteoglycan: the influence of LDL particle size and the relationship to atherosclerosis susceptibility. Atherosclerosis. 1989 Jan;75(1):49–59. doi: 10.1016/0021-9150(89)90206-2. [DOI] [PubMed] [Google Scholar]
- Walker L. N., Bowyer D. E. Endothelial healing in the rabbit aorta and the effect of risk factors for atherosclerosis. Hypercholesterolemia. Arteriosclerosis. 1984 Sep-Oct;4(5):479–488. doi: 10.1161/01.atv.4.5.479. [DOI] [PubMed] [Google Scholar]
- Wolfbauer G., Glick J. M., Minor L. K., Rothblat G. H. Development of the smooth muscle foam cell: uptake of macrophage lipid inclusions. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7760–7764. doi: 10.1073/pnas.83.20.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilversmit D. B. Atherogenesis: a postprandial phenomenon. Circulation. 1979 Sep;60(3):473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]