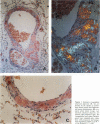
Abstract
The N-terminus of a mutant form of apolipoprotein AI [apoAI] has previously been shown to be the subunit protein of amyloid fibrils in a human kindred with a form of familial amyloid polyneuropathy (FAP, type III) and in a recently reported kindred with a form of non-neuropathic hereditary amyloidosis. In this study, we demonstrate by amino-acid sequence analysis, that a form of vascular amyloidosis occurring in the lungs of aged dogs is derived from a N-terminal fragment of apoAI and that no amino acid substitution is present in this confirmed sequence. This represents the first documentation of apoAI as a precursor for a form of amyloidosis in animals, and provides the first documentation of apoAI as a precursor for amyloid fibrils in a form of age-associated ("senile") amyloidosis. Secondary structure prediction analysis of the N-terminal regions of normal human and dog apoAI indicated a propensity for beta-pleated sheet conformation, and thus amyloidogenesis, in 40 and 45% of the respective sequences. These results suggest that apoAI (like transthyretin) may serve as an amyloid precursor protein for both familial and senile forms of amyloidosis. ApoAI should, therefore, be considered as a potential amyloid precursor when forms of human senile amyloidosis of unknown origin are evaluated.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Benson M. D. Inherited amyloidosis. J Med Genet. 1991 Feb;28(2):73–78. doi: 10.1136/jmg.28.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Erlandsen S. L., Parsons J. A., Burke J. P., Redick J. A., Van Orden D. E., Van Orden L. S. A modification of the unlabeled antibody enzyme method using heterologous antisera for the light microscopic and ultrastructural localization of insulin, glucagon and growth hormone. J Histochem Cytochem. 1975 Sep;23(9):666–677. doi: 10.1177/23.9.1176760. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Shore V. G., Fielding P. E. Lecithin: cholesterol acyltransferase: effects of substrate composition upon enzyme activity. Biochim Biophys Acta. 1972 Aug 11;270(4):513–518. doi: 10.1016/0005-2760(72)90116-6. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Phylogenies constrained by the crossover process as illustrated by human hemoglobins and a thirteen-cycle, eleven-amino-acid repeat in human apolipoprotein A-I. Genetics. 1977 Jul;86(3):623–644. doi: 10.1093/genetics/86.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Rosenthal C. J., Franklin E. C. Amyloid-related serum component (SAA)--studies in acute infections, medullary thyroid carcinoma, and postsurgery. Clin Immunol Immunopathol. 1976 Jul;6(1):83–93. doi: 10.1016/0090-1229(76)90063-5. [DOI] [PubMed] [Google Scholar]
- Higuchi K., Yonezu T., Kogishi K., Matsumura A., Takeshita S., Higuchi K., Kohno A., Matsushita M., Hosokawa M., Takeda T. Purification and characterization of a senile amyloid-related antigenic substance (apoSASSAM) from mouse serum. apoSASSAM is an apoA-II apolipoprotein of mouse high density lipoproteins. J Biol Chem. 1986 Sep 25;261(27):12834–12840. [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Amphiphilic secondary structure: design of peptide hormones. Science. 1984 Jan 20;223(4633):249–255. doi: 10.1126/science.6322295. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Kunisada T., Higuchi K., Aota S., Takeda T., Yamagishi H. Molecular cloning and nucleotide sequence of cDNA for murine senile amyloid protein: nucleotide substitutions found in apolipoprotein A-II cDNA of senescence accelerated mouse (SAM). Nucleic Acids Res. 1986 Jul 25;14(14):5729–5740. doi: 10.1093/nar/14.14.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C. C., Li W. H., Chan L. Structure and expression of dog apolipoprotein A-I, E, and C-I mRNAs: implications for the evolution and functional constraints of apolipoprotein structure. J Lipid Res. 1989 Nov;30(11):1735–1746. [PubMed] [Google Scholar]
- McAdam K. P., Elin R. J., Sipe J. D., Wolff S. M. Changes in human serum amyloid A and C-reactive protein after etiocholanolone-induced inflammation. J Clin Invest. 1978 Feb;61(2):390–394. doi: 10.1172/JCI108949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. C., Dwulet F. E., Liepnieks J., Benson M. D. Variant apolipoprotein AI as a major constituent of a human hereditary amyloid. Biochem Biophys Res Commun. 1988 Oct 31;156(2):762–768. doi: 10.1016/s0006-291x(88)80909-4. [DOI] [PubMed] [Google Scholar]
- Nichols W. C., Gregg R. E., Brewer H. B., Jr, Benson M. D. A mutation in apolipoprotein A-I in the Iowa type of familial amyloidotic polyneuropathy. Genomics. 1990 Oct;8(2):318–323. doi: 10.1016/0888-7543(90)90288-6. [DOI] [PubMed] [Google Scholar]
- Parmelee D. C., Titani K., Ericsson L. H., Eriksen N., Benditt E. P., Walsh K. A. Amino acid sequence of amyloid-related apoprotein (apoSAA1) from human high-density lipoprotein. Biochemistry. 1982 Jul 6;21(14):3298–3303. doi: 10.1021/bi00257a008. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh J. C. Pulmonary amyloidosis in a dog. Vet Pathol. 1988 Jan;25(1):102–104. doi: 10.1177/030098588802500120. [DOI] [PubMed] [Google Scholar]
- Skogen B., Sletten K., Lea T., Natvig J. B. Heterogeneity of human amyloid protein AA and its related serum protein, SAA. Scand J Immunol. 1983 Jan;17(1):83–88. doi: 10.1111/j.1365-3083.1983.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Sletten K., Westermark P., Natvig J. B. Senile cardiac amyloid is related to prealbumin. Scand J Immunol. 1980;12(6):503–506. doi: 10.1111/j.1365-3083.1980.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Van Allen M. W., Frohlich J. A., Davis J. R. Inherited predisposition to generalized amyloidosis. Clinical and pathological study of a family with neuropathy, nephropathy, and peptic ulcer. Neurology. 1969 Jan;19(1):10–25. doi: 10.1212/wnl.19.1.10. [DOI] [PubMed] [Google Scholar]
- Westermark G. T., Sletten K., Westermark P. Massive vascular AA-amyloidosis: a histologically and biochemically distinctive subtype of reactive systemic amyloidosis. Scand J Immunol. 1989 Nov;30(5):605–613. doi: 10.1111/j.1365-3083.1989.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Westermark P., Johnson K. H., Sletten K., Hayden D. W. AA-amyloidosis in dogs: partial amino acid sequence of protein AA and immunohistochemical cross-reactivity with human and cow AA-amyloid. Comp Biochem Physiol B. 1985;82(2):211–215. doi: 10.1016/0305-0491(85)90228-7. [DOI] [PubMed] [Google Scholar]
- Westermark P., Sletten K., Johansson B., Cornwell G. G., 3rd Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2843–2845. doi: 10.1073/pnas.87.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P. The heterogeneity of protein AA in secondary (reactive)systemic amyloidosis. Biochim Biophys Acta. 1982 Feb 4;701(1):19–23. doi: 10.1016/0167-4838(82)90306-5. [DOI] [PubMed] [Google Scholar]
- Zschiesche W., Jakob W. Pathology of animal amyloidoses. Pharmacol Ther. 1989;41(1-2):49–83. doi: 10.1016/0163-7258(89)90102-2. [DOI] [PubMed] [Google Scholar]
- van Rijswijk M. H., van Heusden C. W. The potassium permanganate method. A reliable method for differentiating amyloid AA from other forms of amyloid in routine laboratory practice. Am J Pathol. 1979 Oct;97(1):43–58. [PMC free article] [PubMed] [Google Scholar]