Abstract
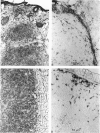
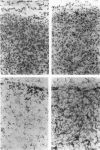
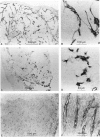
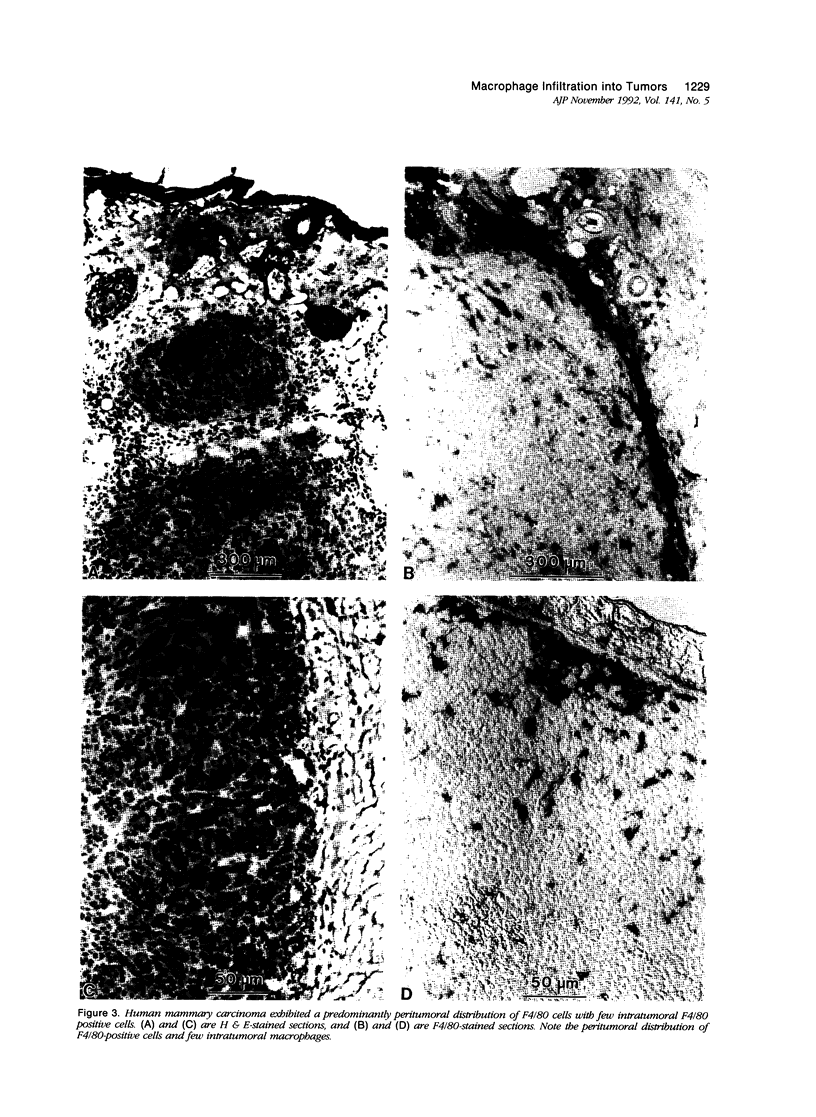
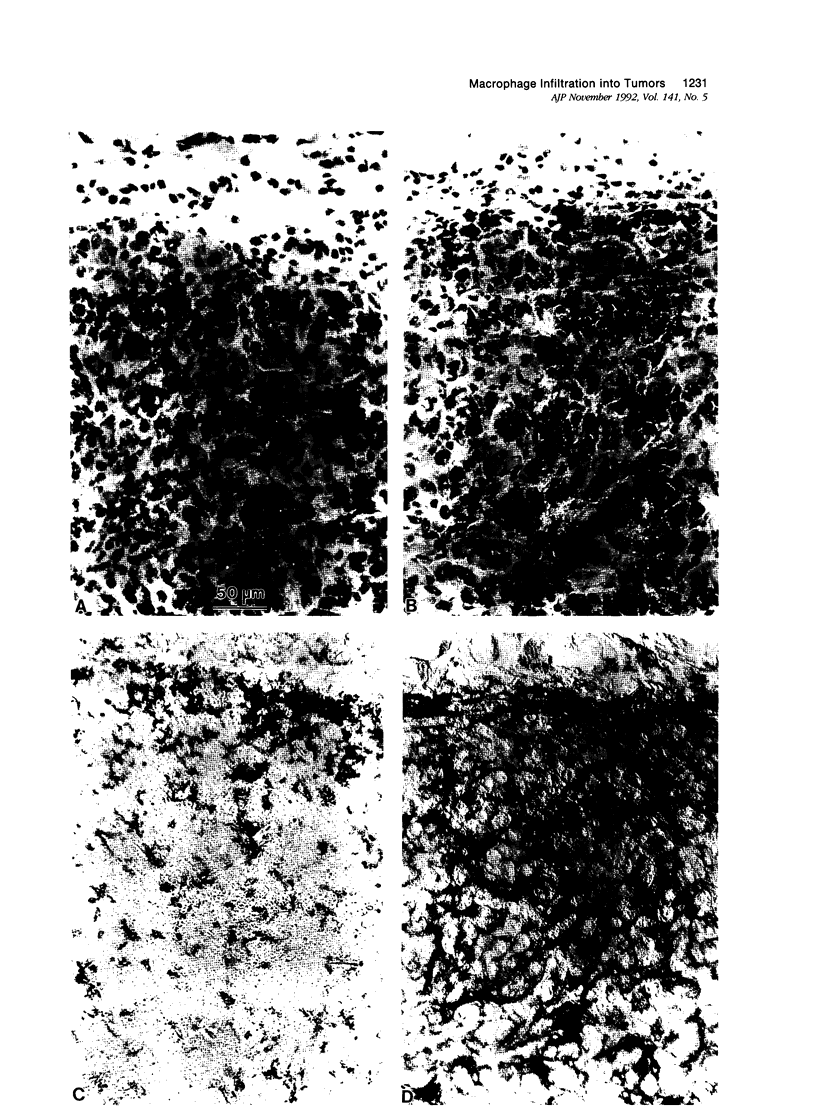
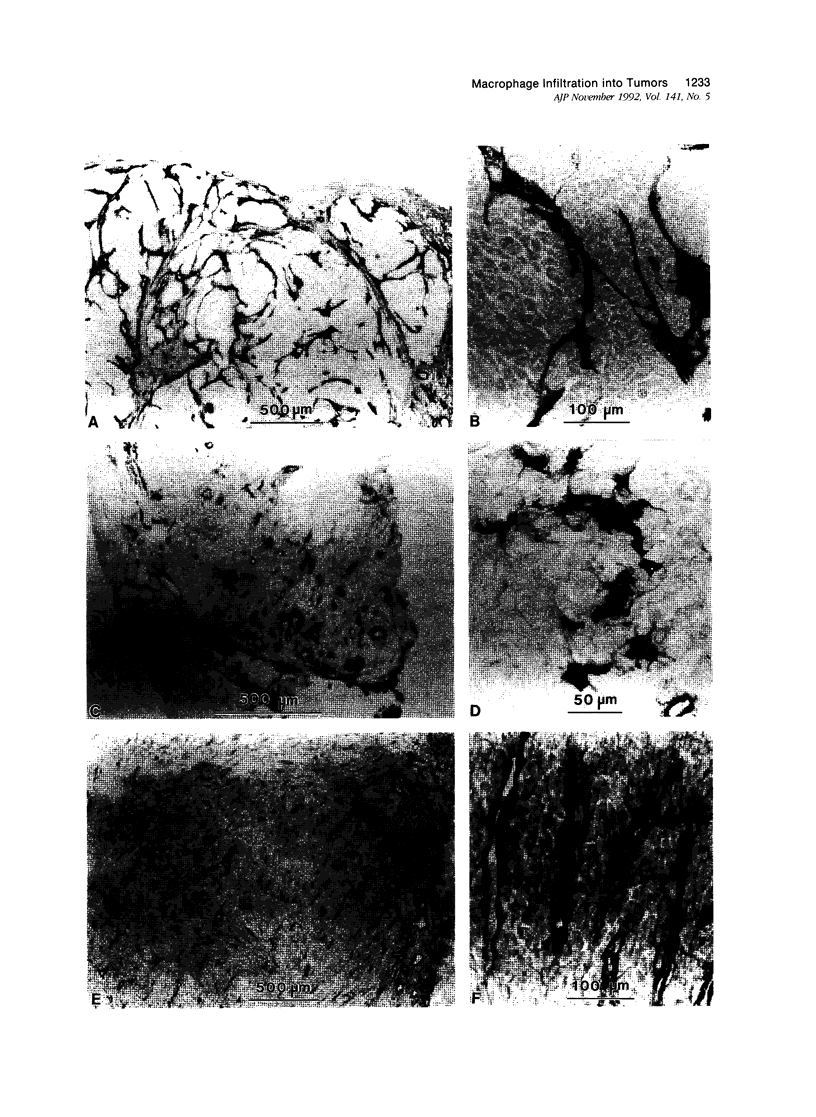
This study determined the distribution pattern of tumor-associated macrophages (TAM) in murine and human neoplasms growing subcutaneously in nude mice. Seven different human neoplasms (cancers of the breast, kidney, colon, prostate, lung, and skin, and a melanoma) and five different murine neoplasms (carcinomas of the lung, colon, and kidney, melanoma, and fibrosarcoma) were injected into nude mice. The murine tumors also were injected into syngeneic mice. Tumor-associated macrophages in small and large tumors were studied immunohistochemically by the use of several antibodies, including the macrophage-specific F4/80. The pattern of TAM distribution differed between mouse and human tumors. Regardless of histologic classification, TAM were uniformly distributed throughout all the murine neoplasms growing in syngeneic or nude mice. In the human neoplasms, TAM were found on the periphery of the lesions and in association with fibrous septae. The distribution of TAM in murine and human tumors was associated with a pattern of vascularization as determined by antibodies to basement membrane collagen type IV. Because the pattern of TAM distribution in neoplasms influences their antitumor activity, the data question the validity of the nude mouse model for the study of macrophage infiltration into human neoplasms.
Full text
PDF

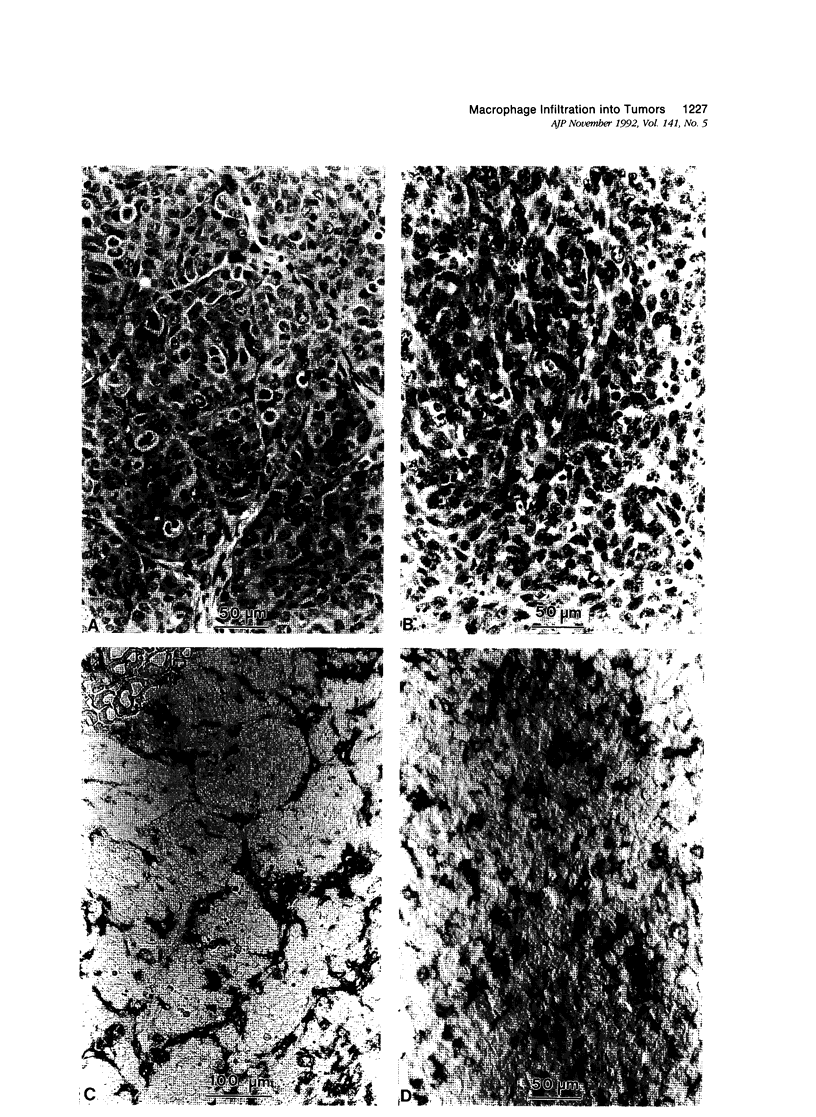





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bernstein I. D., Zbar B., Rapp H. J. Impaired inflammatory response in tumor-bearing guinea pigs. J Natl Cancer Inst. 1972 Dec;49(6):1641–1647. doi: 10.1093/jnci/49.6.1641. [DOI] [PubMed] [Google Scholar]
- Bucana C., Hoyer L. C., Hobbs B., Breesman S., McDaniel M., Hanna M. G., Jr Morphological evidence for the translocation of lysosomal organelles from cytotoxic macrophages into the cytoplasm of tumor target cells. Cancer Res. 1976 Dec;36(12):4444–4458. [PubMed] [Google Scholar]
- Bugelski P. J., Corwin S. P., North S. M., Kirsh R. L., Nicolson G. L., Poste G. Macrophage content of spontaneous metastases at different stages of growth. Cancer Res. 1987 Aug 1;47(15):4141–4145. [PubMed] [Google Scholar]
- Bugelski P. J., Kirsh R. L., Poste G. New histochemical method for measuring intratumoral macrophages and macrophage recruitment into experimental metastases. Cancer Res. 1983 Nov;43(11):5493–5501. [PubMed] [Google Scholar]
- Bugelski P. J., Kirsh R. L., Sowinski J. M., Poste G. Changes in the macrophage content of lung metastases at different stages in tumor growth. Am J Pathol. 1985 Mar;118(3):419–424. [PMC free article] [PubMed] [Google Scholar]
- Bugelski P. J., Kirsh R., Buscarino C., Corwin S. P., Poste G. Recruitment of exogenous macrophages into metastases at different stages of tumor growth. Cancer Immunol Immunother. 1987;24(2):93–98. doi: 10.1007/BF00205584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delabie J., de Wolf-Peeters C., van den Oord J. J., Desmet V. J. Differential expression of the calcium-binding proteins MRP8 and MRP14 in granulomatous conditions: an immunohistochemical study. Clin Exp Immunol. 1990 Jul;81(1):123–126. doi: 10.1111/j.1365-2249.1990.tb05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Lawler E. M. Macrophage content and immunogenicity of C57BL/6J and BALB/cByJ methylcholanthrene-induced sarcomas. Int J Cancer. 1980 Dec 15;26(6):831–835. doi: 10.1002/ijc.2910260618. [DOI] [PubMed] [Google Scholar]
- Evans R. Macrophages and neoplasms: new insights and their implication in tumor immunobiology. Cancer Metastasis Rev. 1982;1(3):227–239. doi: 10.1007/BF00046829. [DOI] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B., Jacob H., Gaillard J. A., Jacob F. Antiinflammatory effects of murine malignant cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4052–4056. doi: 10.1073/pnas.71.10.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J. Macrophages and metastasis--a biological approach to cancer therapy. Cancer Res. 1985 Oct;45(10):4714–4726. [PubMed] [Google Scholar]
- Fidler I. J. Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metastasis Rev. 1986;5(1):29–49. doi: 10.1007/BF00049529. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Schroit A. J. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988 Nov 15;948(2):151–173. doi: 10.1016/0304-419x(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Discrimination between neoplastic and non-neoplastic cells in vitro by activated macrophages. J Natl Cancer Inst. 1974 Nov;53(5):1487–1492. doi: 10.1093/jnci/53.5.1487. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder I., Aherne W., Stewart J., Sainsbury R. Macrophage infiltration of breast tumours: a prospective study. J Clin Pathol. 1977 Jun;30(6):563–568. doi: 10.1136/jcp.30.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Starkey P. M., Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985 Mar 1;161(3):475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwen E. G., Kurzman I. D., Rosenthal R. C., Smith B. W., Manley P. A., Roush J. K., Howard P. E. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989 Jun 21;81(12):935–938. doi: 10.1093/jnci/81.12.935. [DOI] [PubMed] [Google Scholar]
- Murray J. L., Kleinerman E. S., Cunningham J. E., Tatom J. R., Andrejcio K., Lepe-Zuniga J., Lamki L. M., Rosenblum M. G., Frost H., Gutterman J. U. Phase I trial of liposomal muramyl tripeptide phosphatidylethanolamine in cancer patients. J Clin Oncol. 1989 Dec;7(12):1915–1925. doi: 10.1200/JCO.1989.7.12.1915. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman S. J., Sorkin E. Cell-specific defect in monocyte function during tumor growth. J Natl Cancer Inst. 1976 Jul;57(1):135–140. doi: 10.1093/jnci/57.1.135. [DOI] [PubMed] [Google Scholar]
- Normann S. J. Macrophage infiltration and tumor progression. Cancer Metastasis Rev. 1985;4(4):277–291. doi: 10.1007/BF00048093. [DOI] [PubMed] [Google Scholar]
- Normann S. J., Schardt M. C., Sorkin E. Macrophage inflammatory responses in rats and mice with autochthonous and transplanted tumors induced by 3-methylcholanthrene. J Natl Cancer Inst. 1984 Jan;72(1):175–184. doi: 10.1093/jnci/72.1.175. [DOI] [PubMed] [Google Scholar]
- Normann S. J., Schardt M., Sorkin E. Alteration of macrophage function in AKR leukemia. J Natl Cancer Inst. 1981 Jan;66(1):157–162. [PubMed] [Google Scholar]
- Normann S. J., Schardt M., Sorkin E. Anti-inflammatory effect of spontaneous lymphoma in SJL/J mice. J Natl Cancer Inst. 1979 Sep;63(3):825–833. doi: 10.1093/jnci/63.3.825. [DOI] [PubMed] [Google Scholar]
- Odink K., Cerletti N., Brüggen J., Clerc R. G., Tarcsay L., Zwadlo G., Gerhards G., Schlegel R., Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987 Nov 5;330(6143):80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Kerbel R. S. An assessment of intratumor phagocytic and surface marker-bearing cells in a series of autochthonous and early passaged chemically induced murine sarcomas. J Natl Cancer Inst. 1976 Nov;57(5):1157–1167. doi: 10.1093/jnci/57.5.1157. [DOI] [PubMed] [Google Scholar]
- Rubin J. T., Elwood L. J., Rosenberg S. A., Lotze M. T. Immunohistochemical correlates of response to recombinant interleukin-2-based immunotherapy in humans. Cancer Res. 1989 Dec 15;49(24 Pt 1):7086–7092. [PubMed] [Google Scholar]
- Russell P. J., Philips J., Allan W., Hume D. A. Antigenic variation and macrophage infiltration of human bladder tumors xenografted into nude mice. J Leukoc Biol. 1988 Apr;43(4):335–342. doi: 10.1002/jlb.43.4.335. [DOI] [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y. Nature, function and distribution of inflammatory cells in regressing and progressing Moloney sarcomas. J Reticuloendothel Soc. 1977 Aug;22(2):159–168. [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y., Pace J. L. Evidence for mononuclear phagocytes in solid neoplasms and appraisal of their nonspecific cytotoxic capabilities. Contemp Top Immunobiol. 1980;10:143–166. doi: 10.1007/978-1-4684-3677-8_6. [DOI] [PubMed] [Google Scholar]
- Schroit A. J., Hart I. R., Madsen J., Fidler I. J. Selective delivery of drugs encapsulated in liposomes: natural targeting to macrophages involved in various disease states. J Biol Response Mod. 1983;2(2):97–100. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Blaylock B. L., Weinstein P. Effects of neoplasms on inflammation: depression of macrophage accumulation after tumor implantation. J Immunol. 1976 Mar;116(3):585–589. [PubMed] [Google Scholar]
- Svennevig J. L., Svaar H. Content and distribution of macrophages and lymphocytes in solid malignant human tumours. Int J Cancer. 1979 Dec 15;24(6):754–758. doi: 10.1002/ijc.2910240609. [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Key M., Fidler I. J. Macrophage content of metastatic and nonmetastatic rodent neoplasms. J Immunol. 1981 Jun;126(6):2245–2248. [PubMed] [Google Scholar]
- Underwood J. C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974 Dec;30(6):538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaage J., Lindblad W. J. Production of collagen type I by mouse peritoneal macrophages. J Leukoc Biol. 1990 Sep;48(3):274–280. doi: 10.1002/jlb.48.3.274. [DOI] [PubMed] [Google Scholar]
- Vaage J., Pepin K. G. Morphological observations during developing concomitant immunity against a C3H/He mammary tumor. Cancer Res. 1985 Feb;45(2):659–666. [PubMed] [Google Scholar]
- Wei W. Z., Ratner S., Fulton A. M., Heppner G. H. Inflammatory infiltrates of experimental mammary cancers. Biochim Biophys Acta. 1986 Aug 5;865(1):13–26. doi: 10.1016/0304-419x(86)90010-7. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Gollahon K. A. Detection and quantitation of macrophage infiltration into primary human tumors with the use of cell-surface markers. J Natl Cancer Inst. 1977 Oct;59(4):1081–1087. doi: 10.1093/jnci/59.4.1081. [DOI] [PubMed] [Google Scholar]