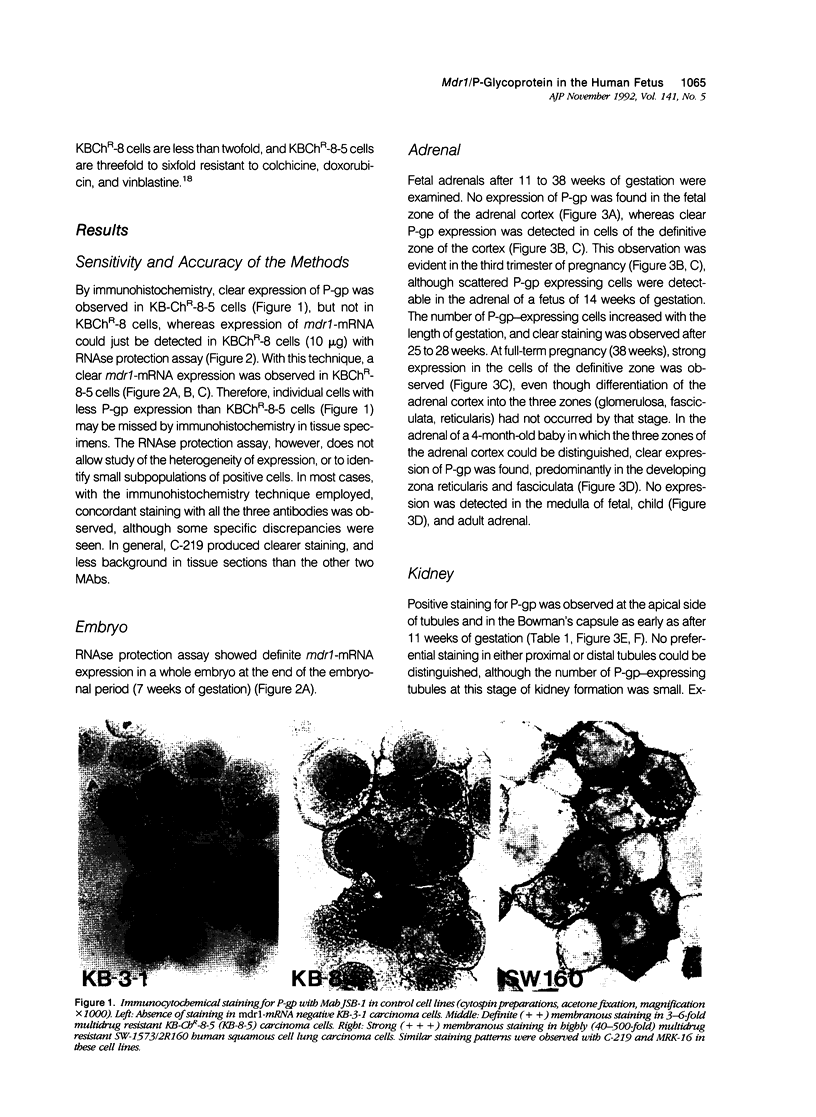
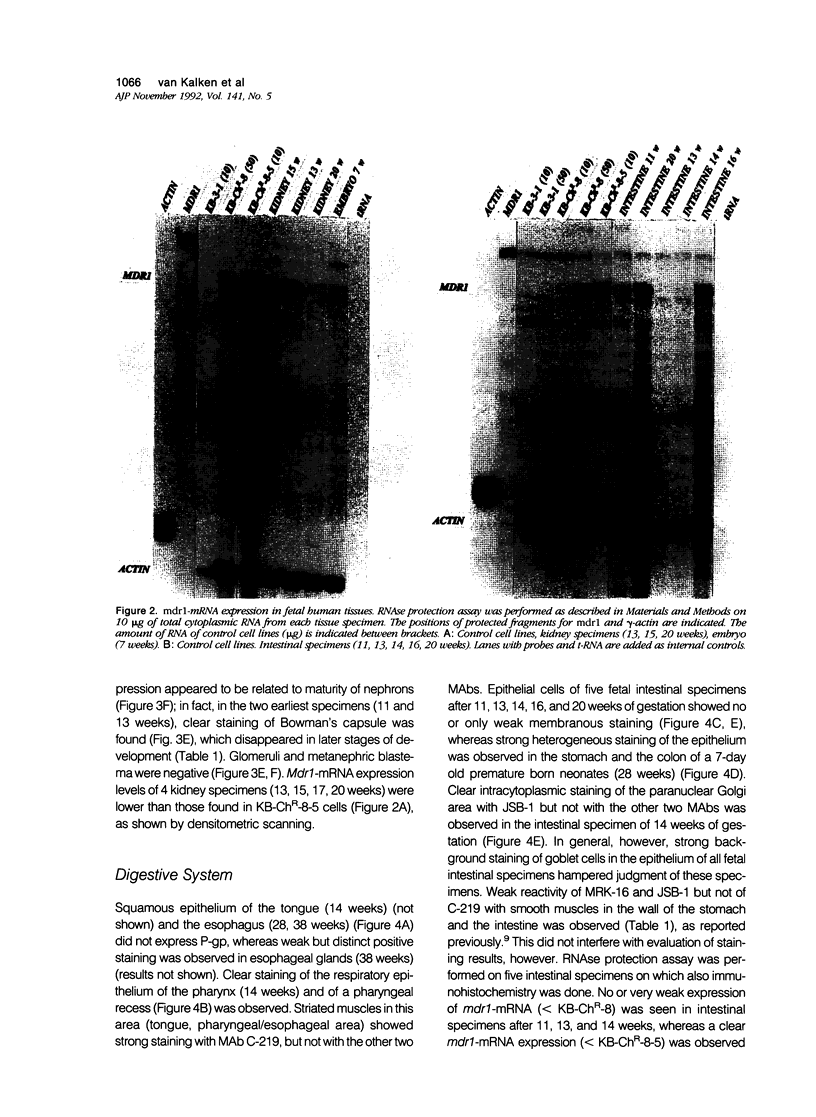
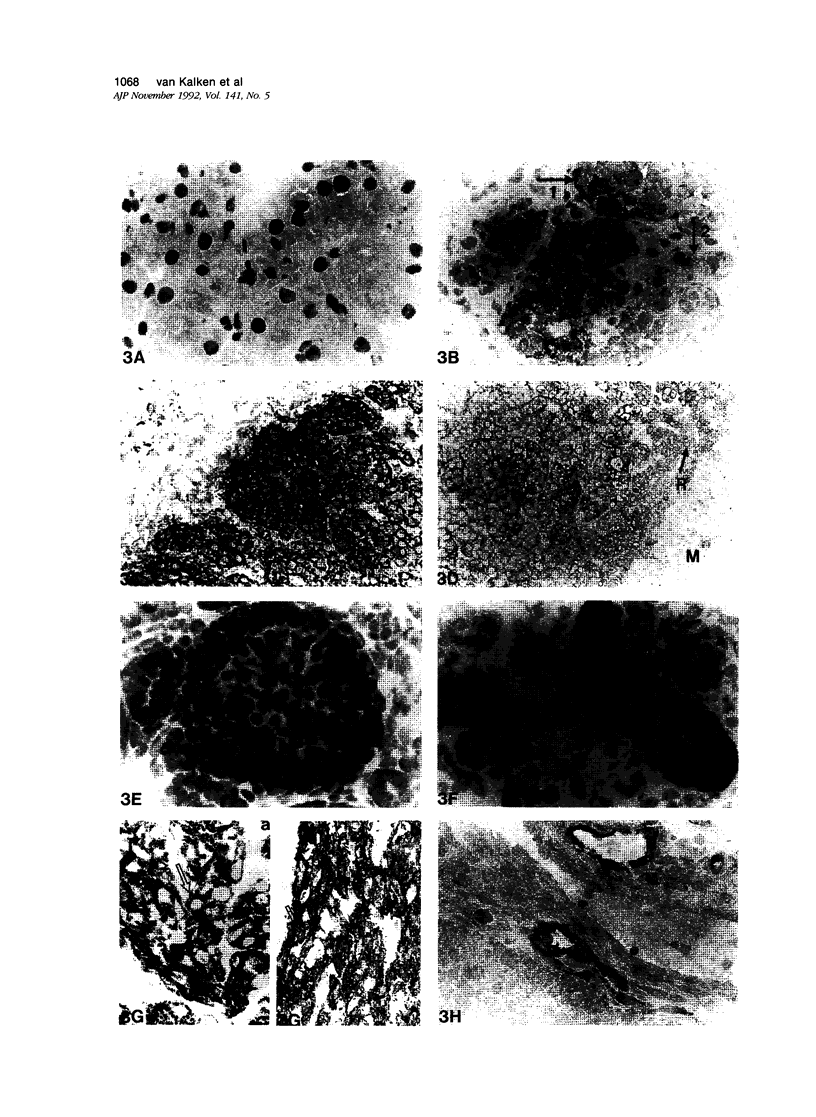
Abstract
P-glycoprotein, a transmembrane protein associated with multidrug resistance in cancer cells, is also expressed in normal tissues. To get more insight into the physiologic role of mdr1/P-glycoprotein, we investigated its expression in human fetal tissues after 7 to 38 weeks of gestation by an immunohistochemical technique, using three different monoclonal antibodies, and by a sensitive RNAse protection assay. Expression of mdr1-mRNA could already be demonstrated in the embryonal phase of human development, after 7 weeks of gestation. Comparing the adult with the fetal tissue distribution, differences were found in specific organs, such as adrenal, intestine, respiratory epithelium, and brain capillaries. In the fetal zone cells of the fetal adrenal cortex no staining was observed by immunohistochemistry, whereas the definitive zone showed increasing expression throughout gestation. Prenatal intestine did not show staining of the epithelium, although definite mdr1-mRNA expression was observed in late specimens. Interestingly, respiratory epithelium of main bronchi and pharynx, not expressing P-gp in adults, did stain positive. Expression of P-gp in brain capillaries was not observed before the third trimester of pregnancy, whereas in kidney and liver, mdr1-mRNA expression and staining for P-glycoprotein were detected in early fetal life (11 to 14 weeks). These findings suggest a pivotal role of P-glycoprotein in physiology of various organs already in early phases of human development and may help to identify its physiologic substrates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Bradley G., Georges E., Ling V. Sex-dependent and independent expression of the P-glycoprotein isoforms in Chinese hamster. J Cell Physiol. 1990 Dec;145(3):398–408. doi: 10.1002/jcp.1041450303. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Bradley G., Thorner P., Haddad G., Gallie B. L., Ling V. A sensitive method for immunocytochemical detection of P-glycoprotein in multidrug-resistant human ovarian carcinoma cell lines. Lab Invest. 1988 Dec;59(6):870–875. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Casals D., Rittman-Grauer L., Biedler J. L., Melamed M. R., Bertino J. R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989 Jan;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angio G. J., Breslow N., Beckwith J. B., Evans A., Baum H., deLorimier A., Fernbach D., Hrabovsky E., Jones B., Kelalis P. Treatment of Wilms' tumor. Results of the Third National Wilms' Tumor Study. Cancer. 1989 Jul 15;64(2):349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Doody K. M., Carr B. R., Rainey W. E., Byrd W., Murry B. A., Strickler R. C., Thomas J. L., Mason J. I. 3 beta-hydroxysteroid dehydrogenase/isomerase in the fetal zone and neocortex of the human fetal adrenal gland. Endocrinology. 1990 May;126(5):2487–2492. doi: 10.1210/endo-126-5-2487. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Functional role for the 170- to 180-kDa glycoprotein specific to drug-resistant tumor cells as revealed by monoclonal antibodies. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7785–7789. doi: 10.1073/pnas.83.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Mierau G. W., Beckwith J. B., Weeks D. A. Ultrastructure and histogenesis of the renal tumors of childhood: an overview. Ultrastruct Pathol. 1987;11(2-3):313–333. doi: 10.3109/01913128709048329. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Human fetal serum cortisol levels related to gestational age: evidence of a midgestational fall and a steep late gestational rise, independent of sex or mode of delivery. Am J Obstet Gynecol. 1982 Oct 1;144(3):276–282. doi: 10.1016/0002-9378(82)90579-8. [DOI] [PubMed] [Google Scholar]
- Nelson H. P., Kuhn R. W., Deyman M. E., Jaffe R. B. Human fetal adrenal definitive and fetal zone metabolism of pregnenolone and corticosterone: alternate biosynthetic pathways and absence of detectable aldosterone synthesis. J Clin Endocrinol Metab. 1990 Mar;70(3):693–698. doi: 10.1210/jcem-70-3-693. [DOI] [PubMed] [Google Scholar]
- Nishida T., Gatmaitan Z., Che M., Arias I. M. Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6590–6594. doi: 10.1073/pnas.88.15.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe G. J., Albrecht E. D. Regulation of the primate fetal adrenal cortex. Endocr Rev. 1990 Feb;11(1):151–176. doi: 10.1210/edrv-11-1-151. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Schinkel A. H., Roelofs E. M., Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res. 1991 May 15;51(10):2628–2635. [PubMed] [Google Scholar]
- Serón-Ferré M., Lawrence C. C., Siiteri P. K., Jaffe R. B. Steroid production by definitive and fetal zones of the human fetal adrenal gland. J Clin Endocrinol Metab. 1978 Sep;47(3):603–609. doi: 10.1210/jcem-47-3-603. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Cardarelli C., Hwang J., Cornwell M., Richert N., Ishii S., Pastan I., Gottesman M. M. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986 Jun 15;261(17):7762–7770. [PubMed] [Google Scholar]
- Simonian M. H., Capp M. W. Characterization of steroidogenesis in cell cultures of the human fetal adrenal cortex: comparison of definitive zone and fetal zone cells. J Clin Endocrinol Metab. 1984 Oct;59(4):643–651. doi: 10.1210/jcem-59-4-643. [DOI] [PubMed] [Google Scholar]
- Sugawara I., Hamada H., Nakahama M., Okamoto S., Tsuruo T., Mori S. Further characterization of the human adrenal-derived P-glycoprotein recognized by monoclonal antibody MRK 16 reacting with only human P-glycoprotein. Jpn J Cancer Res. 1989 Dec;80(12):1199–1205. doi: 10.1111/j.1349-7006.1989.tb01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara I., Kataoka I., Morishita Y., Hamada H., Tsuruo T., Itoyama S., Mori S. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res. 1988 Apr 1;48(7):1926–1929. [PubMed] [Google Scholar]
- Sugawara I., Kataoka I., Morishita Y., Hamada H., Tsuruo T., Itoyama S., Mori S. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res. 1988 Apr 1;48(7):1926–1929. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989 Feb;37(2):159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Ten Houte de Lange T., Kooiman P. M., Van der Velde-Koerts T., Borst P. The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J. 1987 Nov;6(11):3325–3331. doi: 10.1002/j.1460-2075.1987.tb02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- van Kalken C. K., Pinedo H. M., Giaccone G. Multidrug resistance from the clinical point of view. Eur J Cancer. 1991;27(11):1481–1486. doi: 10.1016/0277-5379(91)90036-d. [DOI] [PubMed] [Google Scholar]
- van Kalken C. K., van der Valk P., Hadisaputro M. M., Pieters R., Broxterman H. J., Kuiper C. M., Scheffer G. L., Veerman A. J., Meyer C. J., Scheper R. J. Differentiation dependent expression of P-glycoprotein in the normal and neoplastic human kidney. Ann Oncol. 1991 Jan;2(1):55–62. doi: 10.1093/oxfordjournals.annonc.a057825. [DOI] [PubMed] [Google Scholar]
- van der Valk P., van Kalken C. K., Ketelaars H., Broxterman H. J., Scheffer G., Kuiper C. M., Tsuruo T., Lankelma J., Meijer C. J., Pinedo H. M. Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein molecule. Ann Oncol. 1990;1(1):56–64. [PubMed] [Google Scholar]