Abstract
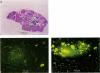
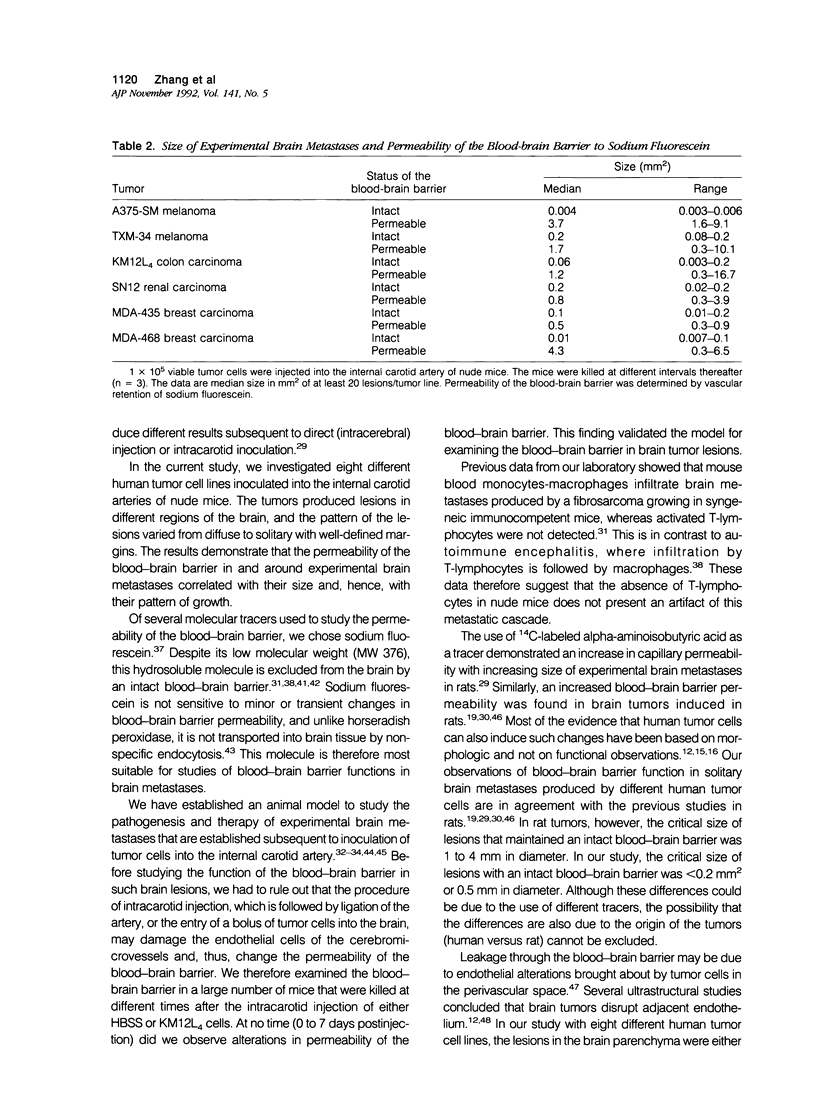
This study clarified whether and when the blood-brain barrier in experimental brain metastases is impaired by using hydrosoluble sodium fluorescein (MW 376) as a blood-brain barrier function indicator. Cells from eight human tumor lines (four melanomas, two breast carcinomas, one colon carcinoma, and one renal carcinoma) were inoculated into the internal carotid artery of nude mice. Brain metastases at different stages of development were sampled and the permeability of the blood-brain barrier around the metastases determined. Histologic examination showed two patterns of tumor growth. In the first, tumor cells formed isolated, well-defined nodules in the parenchyma of the brain. In lesions smaller than 0.2 mm2, the blood-brain barrier was intact. In the second, small diffuse nests of tumor cells were distributed throughout the brain parenchyma. The blood-brain barrier was intact until the small tumor cell colonies coalesced to form large tumor masses. These results suggest that the permeability of the blood-brain barrier varies among different experimental brain metastases and that its function is related to the growth pattern and size of the lesions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballinger W. E., Jr, Schimpff R. D. An experimental model for cerebral metastasis: preliminary light and ultrastructural studies. J Neuropathol Exp Neurol. 1979 Jan;38(1):19–34. doi: 10.1097/00005072-197901000-00003. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Aluminum-induced neurotoxicity: alterations in membrane function at the blood-brain barrier. Neurosci Biobehav Rev. 1989 Spring;13(1):47–53. doi: 10.1016/s0149-7634(89)80051-x. [DOI] [PubMed] [Google Scholar]
- Bauer H. C., Tontsch U., Amberger A., Bauer H. Gamma-glutamyl-transpeptidase (GGTP) and NA+K(+)-ATPase activities in different subpopulations of cloned cerebral endothelial cells: responses to glial stimulation. Biochem Biophys Res Commun. 1990 Apr 16;168(1):358–363. doi: 10.1016/0006-291x(90)91716-6. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Lightman S. L. The blood-brain interface. Eye (Lond) 1990;4(Pt 2):249–254. doi: 10.1038/eye.1990.36. [DOI] [PubMed] [Google Scholar]
- Coomber B. L., Stewart P. A., Hayakawa K., Farrell C. L., Del Maestro R. F. Quantitative morphology of human glioblastoma multiforme microvessels: structural basis of blood-brain barrier defect. J Neurooncol. 1987;5(4):299–307. doi: 10.1007/BF00148386. [DOI] [PubMed] [Google Scholar]
- Debbage P. L., Gabius H. J., Bise K., Marguth F. Cellular glycoconjugates and their potential endogenous receptors in the cerebral microvasculature of man: a glycohistochemical study. Eur J Cell Biol. 1988 Aug;46(3):425–434. [PubMed] [Google Scholar]
- Dietrich W. D., Busto R., Halley M., Valdes I. The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J Neuropathol Exp Neurol. 1990 Sep;49(5):486–497. doi: 10.1097/00005072-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Felgenhauer K. The blood-brain barrier redefined. J Neurol. 1986 Aug;233(4):193–194. doi: 10.1007/BF00314016. [DOI] [PubMed] [Google Scholar]
- Fenstermacher J., Gross P., Sposito N., Acuff V., Pettersen S., Gruber K. Structural and functional variations in capillary systems within the brain. Ann N Y Acad Sci. 1988;529:21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- Freeman A. I., Fenstermacher J., Shapiro W., Kemshead J., Chasin M., Colvin O. M., Diksic M., Finley J., Hertler A., Levin V. Forbeck forum on improved drug delivery to brain tumors. Sel Cancer Ther. 1990 Fall;6(3):109–118. doi: 10.1089/sct.1990.6.109. [DOI] [PubMed] [Google Scholar]
- Front D., Israel O., Kohn S., Nir I. The blood-tissue barrier of human brain tumors: correlation of scintigraphic and ultrastructural findings: concise communication. J Nucl Med. 1984 Apr;25(4):461–465. [PubMed] [Google Scholar]
- Genka S., Deutsch J., Stahle P. L., Shetty U. H., John V., Robinson C., Rapoport S. I., Greig N. H. Brain and plasma pharmacokinetics and anticancer activities of cyclophosphamide and phosphoramide mustard in the rat. Cancer Chemother Pharmacol. 1990;27(1):1–7. doi: 10.1007/BF00689268. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W. Endothelial cell-astrocyte interactions. A cellular model of the blood-brain barrier. Ann N Y Acad Sci. 1988;529:31–39. doi: 10.1111/j.1749-6632.1988.tb51417.x. [DOI] [PubMed] [Google Scholar]
- Gregoire N. The blood-brain barrier. J Neuroradiol. 1989;16(3):238–250. [PubMed] [Google Scholar]
- Greig N. H. Optimizing drug delivery to brain tumors. Cancer Treat Rev. 1987 Mar;14(1):1–28. doi: 10.1016/0305-7372(87)90048-x. [DOI] [PubMed] [Google Scholar]
- Greig N. H., Soncrant T. T., Shetty H. U., Momma S., Smith Q. R., Rapoport S. I. Brain uptake and anticancer activities of vincristine and vinblastine are restricted by their low cerebrovascular permeability and binding to plasma constituents in rat. Cancer Chemother Pharmacol. 1990;26(4):263–268. doi: 10.1007/BF02897227. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Ushio Y., Hayakawa T., Yamada K., Mogami H. Changes of the blood-brain barrier in experimental metastatic brain tumors. J Neurosurg. 1983 Aug;59(2):304–310. doi: 10.3171/jns.1983.59.2.0304. [DOI] [PubMed] [Google Scholar]
- Hirano A., Zimmerman H. M. Fenestrated blood vessels in a metastatic renal carcinoma in the brain. Lab Invest. 1972 Apr;26(4):465–468. [PubMed] [Google Scholar]
- Iannotti F., Fieschi C., Alfano B., Picozzi P., Mansi L., Pozzilli C., Punzo A., Del Vecchio G., Lenzi G. L., Salvatore M. Simplified, noninvasive PET measurement of blood-brain barrier permeability. J Comput Assist Tomogr. 1987 May-Jun;11(3):390–397. doi: 10.1097/00004728-198705000-00004. [DOI] [PubMed] [Google Scholar]
- Johansson B. B. The physiology of the blood-brain barrier. Adv Exp Med Biol. 1990;274:25–39. doi: 10.1007/978-1-4684-5799-5_2. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Schürer L., Goetz A., Kempski O., Schmucker B., Baethmann A. An improved closed cranial window technique for investigation of blood-brain barrier function and cerebral vasomotor control in the rat. Int J Microcirc Clin Exp. 1990 Nov;9(4):369–383. [PubMed] [Google Scholar]
- Kohn S., Front D., Nir I. Blood-brain barrier permeability of human gliomas as determined by quantitation of cytoplasmic vesicles of the capillary endothelium and scintigraphic findings. Cancer Invest. 1989;7(4):313–321. doi: 10.3109/07357908909039856. [DOI] [PubMed] [Google Scholar]
- Malmgren L. T., Olsson Y. Differences between the peripheral and the central nervous system in permeability to sodium fluorescein. J Comp Neurol. 1980 May 1;191(1):103–107. doi: 10.1002/cne.901910106. [DOI] [PubMed] [Google Scholar]
- Morikawa K., Walker S. M., Jessup J. M., Fidler I. J. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988 Apr 1;48(7):1943–1948. [PubMed] [Google Scholar]
- Naito S., von Eschenbach A. C., Giavazzi R., Fidler I. J. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986 Aug;46(8):4109–4115. [PubMed] [Google Scholar]
- Nakagawa Y., Fujimoto N., Matsumoto K., Cervós-Navarro J. Morphological changes in acute cerebral ischemia after occlusion and reperfusion in the rat. Adv Neurol. 1990;52:21–27. [PubMed] [Google Scholar]
- Nir I., Levanon D., Iosilevsky G. Permeability of blood vessels in experimental gliomas: uptake of 99mTc-glucoheptonate and alteration in blood-brain barrier as determined by cytochemistry and electron microscopy. Neurosurgery. 1989 Oct;25(4):523–532. [PubMed] [Google Scholar]
- Owman C., Hardebo J. E. Functional heterogeneity of the cerebrovascular endothelium. Brain Behav Evol. 1988;32(2):65–75. doi: 10.1159/000116534. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H., Cancilla P., Frank H. J. Blood-brain barrier: interface between internal medicine and the brain. Ann Intern Med. 1986 Jul;105(1):82–95. doi: 10.7326/0003-4819-105-1-82. [DOI] [PubMed] [Google Scholar]
- SCHEINBERG L. C., EDELMAN F. L., LEVY W. A. IS THE BRAIN "AN IMMUNOLOGICALLY PRIVILEGED SITE"?I. STUDIES BASED ON INTRACEREBRAL TUMOR HOMOTRANSPLANTATION AND ISOTRANSPLANTATION TO SENSITIZED HOSTS. Arch Neurol. 1964 Sep;11:248–264. doi: 10.1001/archneur.1964.00460210026003. [DOI] [PubMed] [Google Scholar]
- Schackert G., Fan D., Nayar R., Fidler I. J. Arrest and retention of multilamellar liposomes in the brain of normal mice or mice bearing experimental brain metastases. Sel Cancer Ther. 1989;5(2):73–79. doi: 10.1089/sct.1989.5.73. [DOI] [PubMed] [Google Scholar]
- Schackert G., Fidler I. J. Development of in vivo models for studies of brain metastasis. Int J Cancer. 1988 Apr 15;41(4):589–594. doi: 10.1002/ijc.2910410419. [DOI] [PubMed] [Google Scholar]
- Schackert G., Fidler I. J. Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 1988 Jun 15;48(12):3478–3484. [PubMed] [Google Scholar]
- Schackert G., Price J. E., Bucana C. D., Fidler I. J. Unique patterns of brain metastasis produced by different human carcinomas in athymic nude mice. Int J Cancer. 1989 Nov 15;44(5):892–897. doi: 10.1002/ijc.2910440524. [DOI] [PubMed] [Google Scholar]
- Schackert G., Price J. E., Zhang R. D., Bucana C. D., Itoh K., Fidler I. J. Regional growth of different human melanomas as metastases in the brain of nude mice. Am J Pathol. 1990 Jan;136(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Schackert G., Simmons R. D., Buzbee T. M., Hume D. A., Fidler I. J. Macrophage infiltration into experimental brain metastases: occurrence through an intact blood-brain barrier. J Natl Cancer Inst. 1988 Sep 7;80(13):1027–1034. doi: 10.1093/jnci/80.13.1027. [DOI] [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., de Waal R. M., Ferrone S., Ruiter D. J. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990 Jun;136(6):1393–1405. [PMC free article] [PubMed] [Google Scholar]
- Shapiro W. R., Shapiro J. R. Principles of brain tumor chemotherapy. Semin Oncol. 1986 Mar;13(1):56–69. [PubMed] [Google Scholar]
- Simmons R. D., Buzbee T. M., Linthicum D. S., Mandy W. J., Chen G., Wang C. Simultaneous visualization of vascular permeability change and leukocyte egress in the central nervous system during autoimmune encephalomyelitis. Acta Neuropathol. 1987;74(2):191–193. doi: 10.1007/BF00692851. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Hayakawa K., Farrell C. L., Del Maestro R. F. Quantitative study of microvessel ultrastructure in human peritumoral brain tissue. Evidence for a blood-brain barrier defect. J Neurosurg. 1987 Nov;67(5):697–705. doi: 10.3171/jns.1987.67.5.0697. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Wiley M. J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981 May;84(1):183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Terada T., Nishiguchi T., Hyoutani G., Miyamoto K., Nakamura Y., Tsuura M., Komai N. [A study on the vascular permeability of brain tumors using dynamic CT]. No Shinkei Geka. 1989 Sep;17(9):841–848. [PubMed] [Google Scholar]
- Vriesendorp F. J., Peagram C., Bigner D. D., Groothuis D. R. Concurrent measurements of blood flow and transcapillary transport in xenotransplanted human gliomas in immunosuppressed rats. J Natl Cancer Inst. 1987 Jul;79(1):123–130. [PubMed] [Google Scholar]
- Yamada K., Ushio Y., Hayakawa T., Kato A., Yamada N., Mogami H. Quantitative autoradiographic measurements of blood-brain barrier permeability in the rat glioma model. J Neurosurg. 1982 Sep;57(3):394–398. doi: 10.3171/jns.1982.57.3.0394. [DOI] [PubMed] [Google Scholar]
- Zagzag D., Goldenberg M., Brem S. Angiogenesis and blood-brain barrier breakdown modulate CT contrast enhancement: an experimental study in a rabbit brain-tumor model. AJR Am J Roentgenol. 1989 Jul;153(1):141–146. doi: 10.2214/ajr.153.1.141. [DOI] [PubMed] [Google Scholar]
- Zhang R. D., Price J. E., Schackert G., Itoh K., Fidler I. J. Malignant potential of cells isolated from lymph node or brain metastases of melanoma patients and implications for prognosis. Cancer Res. 1991 Apr 15;51(8):2029–2035. [PubMed] [Google Scholar]