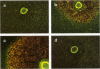
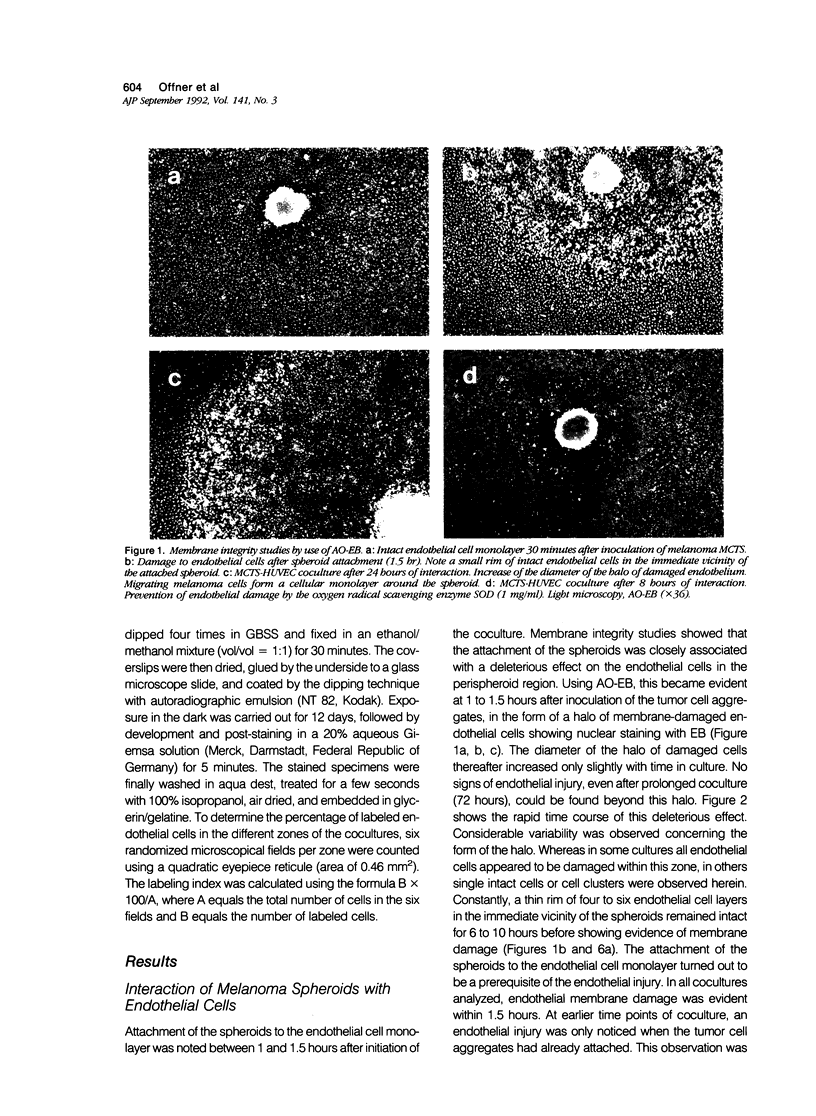
Abstract
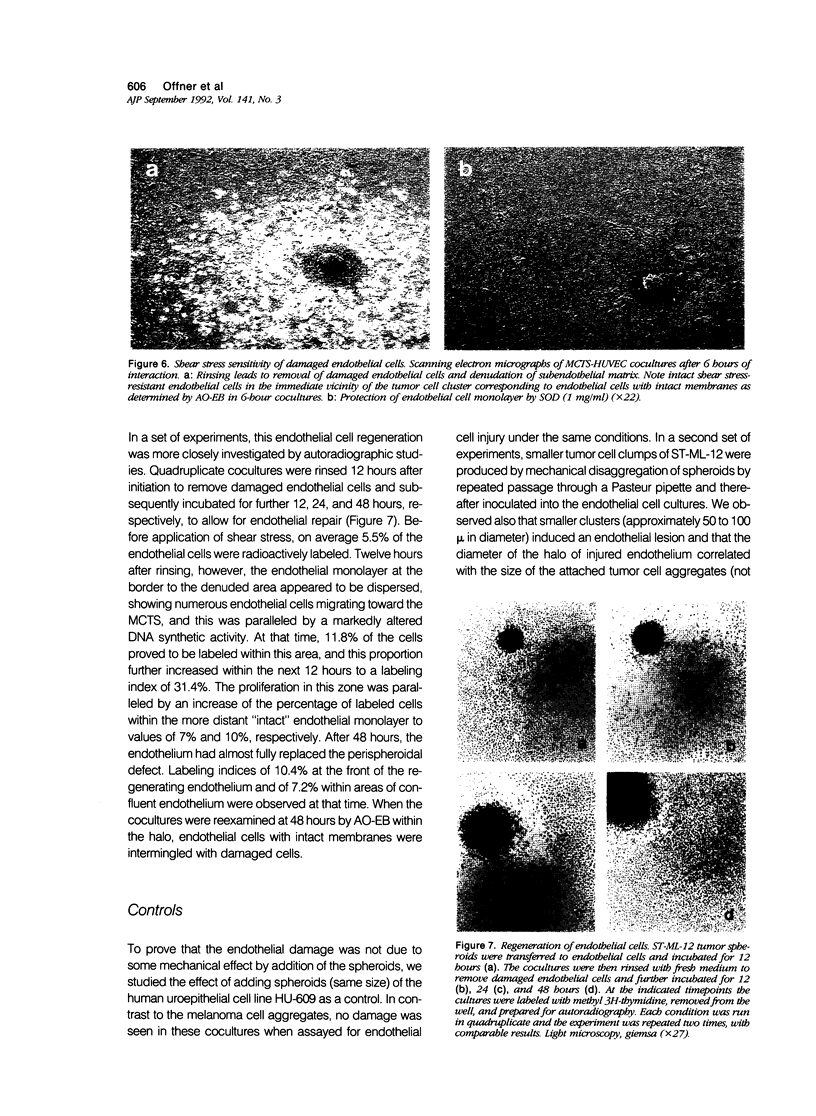
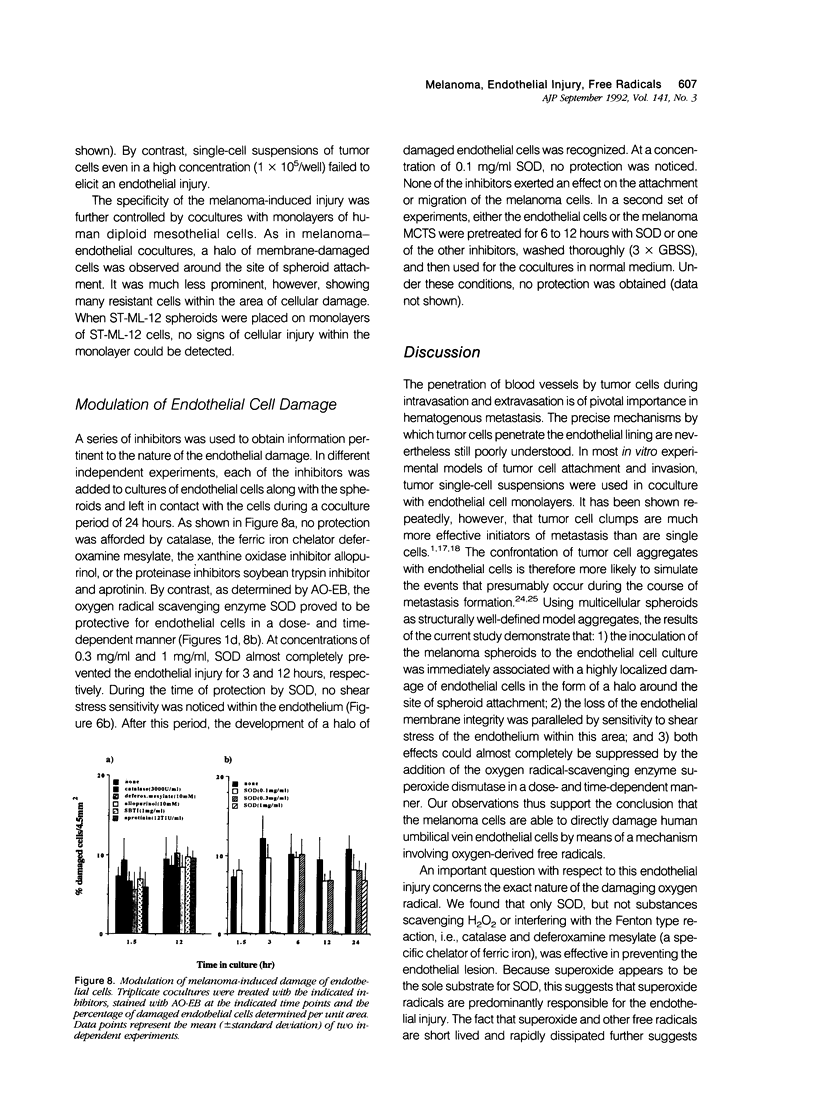
Clinical and experimental observations suggest that tumor-induced endothelial cell injury may be one of several initial events in the establishment of tumor metastases. To test this hypothesis, the authors have analyzed the interaction of malignant melanoma (ST-ML-12) multicenter tumor spheroids with endothelial cell monolayers in a three-dimensional coculture system. After 1.5 hours of interaction, the authors observed a toxic effect on endothelial cells in the perispheroid region. The latter was demonstrated by testing membrane integrity with the fluorescent probes acridine orange/ethidium bromide and resulted in sensitivity to shear stress of the damaged cells. The endothelium then underwent a regenerative cycle to replace the denuded halo. Addition of the oxygen radical-scavenging enzyme superoxide dismutase to the culture medium prevented this endothelial cell damage in a dose-dependent manner for up to 12 hours. By contrast, catalase, deferoxamine mesylate, allopurinol, and the proteinase inhibitors soybean trypsin inhibitor and aprotinin were not protective under the same conditions. The endothelial damage was dependent on the attachment of the spheroids. Medium conditioned by ST-ML-12-spheroids proved to be ineffective. A similar, but less prominent, deleterious effect was seen when human peritoneal mesothelial cells were used in place of the human umbilical vein endothelial cells. Spheroids of the uroepithelial cell line HU-609 were used as control. No toxicity was observed in these cocultures. Melanin biosynthesis is associated with the production of oxygen-derived free radicals. The results suggest a possible implication of these free radicals in metastasis formation of malignant melanoma.
Full text
PDF


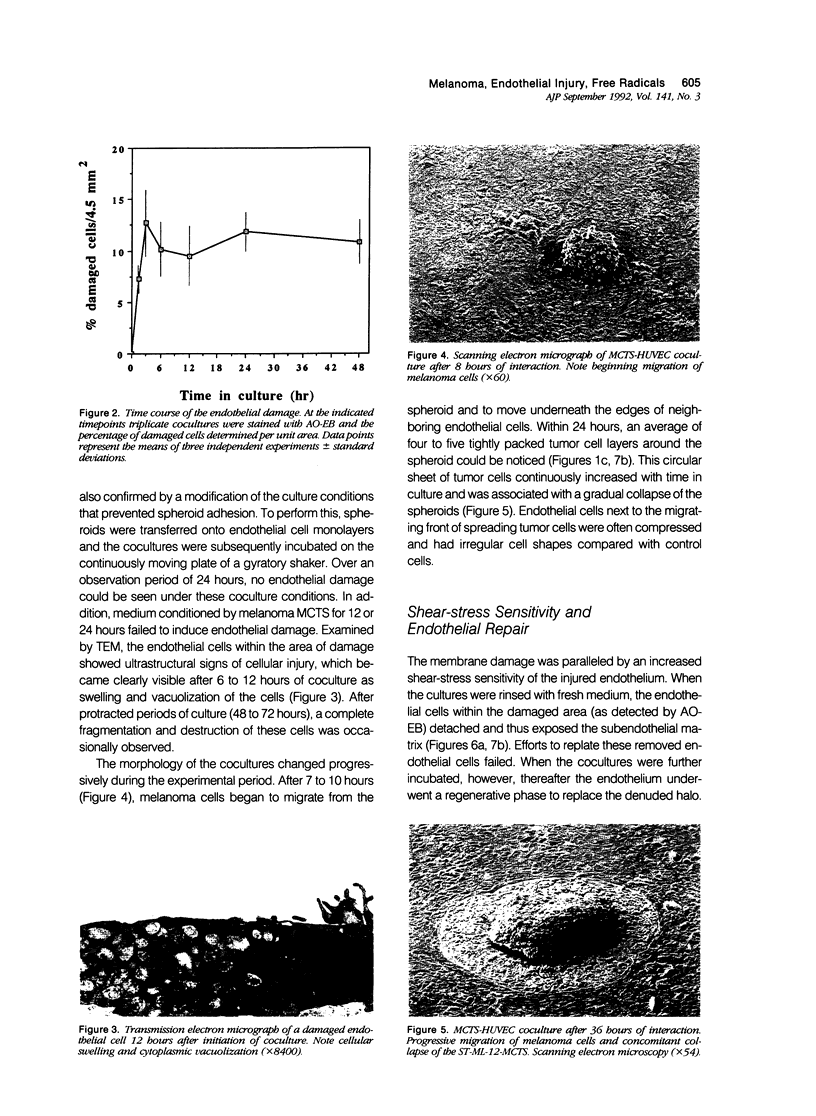



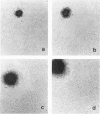
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Orr F. W., Young L. Effects of injury and repair of the pulmonary endothelium on lung metastasis after bleomycin. J Pathol. 1986 Dec;150(4):279–287. doi: 10.1002/path.1711500407. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Young L., Orr F. W. Tumor metastasis after hyperoxic injury and repair of the pulmonary endothelium. Lab Invest. 1987 Jul;57(1):71–77. [PubMed] [Google Scholar]
- Al-Mondhiry H., McGarvey V. Tumor interaction with vascular endothelium. Haemostasis. 1987;17(5):245–253. doi: 10.1159/000215751. [DOI] [PubMed] [Google Scholar]
- Bishop C. T., Mirza Z., Crapo J. D., Freeman B. A. Free radical damage to cultured porcine aortic endothelial cells and lung fibroblasts: modulation by culture conditions. In Vitro Cell Dev Biol. 1985 Apr;21(4):229–236. doi: 10.1007/BF02620934. [DOI] [PubMed] [Google Scholar]
- COMMONER B., TOWNSEND J., PAKE G. E. Free radicals in biological materials. Nature. 1954 Oct 9;174(4432):689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- Crissman J. D., Hatfield J. S., Menter D. G., Sloane B., Honn K. V. Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Res. 1988 Jul 15;48(14):4065–4072. [PubMed] [Google Scholar]
- Dao T. L., Yogo H. Enhancement of pulmonary metastases by x-irradiation in rats bearing mammary cancer. Cancer. 1967 Nov;20(11):2020–2025. doi: 10.1002/1097-0142(196711)20:11<2020::aid-cncr2820201131>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Dejana E., Bertocchi F., Bortolami M. C., Regonesi A., Tonta A., Breviario F., Giavazzi R. Interleukin 1 promotes tumor cell adhesion to cultured human endothelial cells. J Clin Invest. 1988 Oct;82(4):1466–1470. doi: 10.1172/JCI113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik E., Wiltrout R. H., Brunda M. J., Holden H. T., Herberman R. B. Augmentation of metastasis formation by thioglycollate-elicited macrophages. Int J Cancer. 1982 May 15;29(5):575–581. doi: 10.1002/ijc.2910290514. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Honn K. V., Grossi I. M., Diglio C. A., Wojtukiewicz M., Taylor J. D. Enhanced tumor cell adhesion to the subendothelial matrix resulting from 12(S)-HETE-induced endothelial cell retraction. FASEB J. 1989 Sep;3(11):2285–2293. doi: 10.1096/fasebj.3.11.2673900. [DOI] [PubMed] [Google Scholar]
- Imamura F., Horai T., Mukai M., Shinkai K., Akedo H. Potentiation of invasive capacity of rat ascites hepatoma cells by adriamycin. Cancer Res. 1990 Apr 1;50(7):2018–2021. [PubMed] [Google Scholar]
- Kirkpatrick C. J., Bültmann B. D., Gruler H. Interaction between enteroviruses and human endothelial cells in vitro. Alterations in the physical properties of endothelial cell plasma membrane and adhesion of human granulocytes. Am J Pathol. 1985 Jan;118(1):15–25. [PMC free article] [PubMed] [Google Scholar]
- Knüchel R., Feichtinger J., Recktenwald A., Hollweg H. G., Franke P., Jakse G., Rammal E., Hofstädter F. Interactions between bladder tumor cells as tumor spheroids from the cell line J82 and human endothelial cells in vitro. J Urol. 1988 Mar;139(3):640–645. doi: 10.1016/s0022-5347(17)42550-x. [DOI] [PubMed] [Google Scholar]
- Kolb M. J., Bourne W. M. Supravital fluorescent staining of the corneal endothelium with acridine orange and ethidium bromide. Curr Eye Res. 1986 Jul;5(7):485–494. doi: 10.3109/02713688608996370. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Gonzalez R., Nicolson G. L. Metastatic tumor cells adhere preferentially to the extracellular matrix underlying vascular endothelial cells. Int J Cancer. 1980 Nov 15;26(5):639–645. doi: 10.1002/ijc.2910260516. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Nicolson G. L. Interactions of tumor cells with vascular endothelial cell monolayers: a model for metastatic invasion. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5704–5708. doi: 10.1073/pnas.76.11.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri D., Bertomeu M. C., Orr F. W., Bastida E., Sauder D. N., Buchanan M. R. Differential effects of interleukin-1 and formylmethionylleucylphenylalanine on chemotaxis and human endothelium adhesivity for A549 tumor cells. Lab Invest. 1989 Jan;60(1):161–164. [PubMed] [Google Scholar]
- Liotta L. A., Kleinerman J., Saidel G. M. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974 May;34(5):997–1004. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Mukai M., Shinkai K., Komatsu K., Akedo H. Potentiation of invasive capacity of rat ascites hepatoma cells by transforming growth factor-beta. Jpn J Cancer Res. 1989 Feb;80(2):107–110. doi: 10.1111/j.1349-7006.1989.tb02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M., Shinkai K., Tateishi R., Mori Y., Akedo H. Macrophage potentiation of invasive capacity of rat ascites hepatoma cells. Cancer Res. 1987 Apr 15;47(8):2167–2171. [PubMed] [Google Scholar]
- Offner F. A., Ott G., Povey S., Knuechel R., Preisler V., Fuezesi L., Klosterhalfen B., Ruebben H., Hofstaedter F., Kirkpatrick C. J. Characterization of the new bladder cancer cell line HOK-1: expression of transitional, squamous and glandular differentiation patterns. Int J Cancer. 1991 Aug 19;49(1):122–128. doi: 10.1002/ijc.2910490123. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Warner D. J. Effects of neutrophil-mediated pulmonary endothelial injury on the localization and metastasis of circulating Walker carcinosarcoma cells. Invasion Metastasis. 1987;7(3):183–196. [PubMed] [Google Scholar]
- Pezzuto J. M., Shieh H. L., Shaughnessy E., Beattie C. W. Approaches for drug development in treatment of advanced melanoma. Semin Oncol. 1988 Dec;15(6):578–588. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Raz A., Ben-Ze'ev A. Modulation of the metastatic capability in B16 melanoma by cell shape. Science. 1983 Sep 23;221(4617):1307–1310. doi: 10.1126/science.6612347. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Gimbrone M. A., Jr, Bevilacqua M. P. Tumor cell-endothelial interactions. Increased adhesion of human melanoma cells to activated vascular endothelium. Am J Pathol. 1988 Nov;133(2):204–210. [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy S. G., Buchanan M. R., Turple S., Richardson M., Orr F. W. Walker carcinosarcoma cells damage endothelial cells by the generation of reactive oxygen species. Am J Pathol. 1989 Apr;134(4):787–796. [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy S. G., Lafrenie R. M., Buchanan M. R., Podor T. J., Orr F. W. Endothelial cell damage by Walker carcinosarcoma cells is dependent on vitronectin receptor-mediated tumor cell adhesion. Am J Pathol. 1991 Jun;138(6):1535–1543. [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Talbot I. C., Ritchie S., Leighton M., Hughes A. O., Bussey H. J., Morson B. C. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981 Mar;5(2):141–163. doi: 10.1111/j.1365-2559.1981.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Vilien M., Wolf H., Rasmussen F. Immunological characterization of cell lines establishing from malignant and normal human urothelium. Eur J Cancer. 1981 Mar;17(3):321–327. doi: 10.1016/0014-2964(81)90123-7. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Ariav Y., Atzmon R., Fuks Z. Tumor cell attachment to the vascular endothelium and subsequent degradation of the subendothelial extracellular matrix. Exp Cell Res. 1982 Jul;140(1):149–159. doi: 10.1016/0014-4827(82)90166-5. [DOI] [PubMed] [Google Scholar]
- Weiss L., Orr F. W., Honn K. V. Interactions of cancer cells with the microvasculature during metastasis. FASEB J. 1988 Jan;2(1):12–21. doi: 10.1096/fasebj.2.1.3275560. [DOI] [PubMed] [Google Scholar]
- Zamora P. O., Danielson K. G., Hosick H. L. Invasion of endothelial cell monolayers on collagen gels by cells from mammary tumor spheroids. Cancer Res. 1980 Dec;40(12):4631–4639. [PubMed] [Google Scholar]