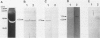Abstract
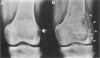
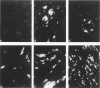
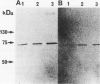
Matrix metalloproteinases play a central role in the catabolism of extracellular matrix macromolecules. Here the authors report that giant cell tumor of bone (GCT) produces two matrix metalloproteinases (MMPs) in zymogen form, which have been identified as proMMP-2 (also known as "72-kDa-progelatinase/type IV procollagenase") and proMMP-3 (prostromelysin). Giant cell tumor is known to consist of two major cell populations, multinucleated giant cells and stromal cells. On several passages of the tumor cells in culture, only stromal cells proliferated. These stromal cells produced proMMP-2 but not proMMP-3. Addition of the conditioned medium of primary GCT culture or human macrophage-conditioned medium to the passaged stromal cells induced the production of proMMP-3. The production of proMMP-3 was also induced by interleukin 1 (IL-1), but not by tumor necrosis factor alpha (TNF alpha). ProMMP-1 (tissue procollagenase) was not detected even after treatment with these stimuli. Immunohistochemical studies have demonstrated that multinucleated giant cells in GCT both produce IL-1 and TNF alpha, suggesting that IL-1 secreted by multinucleated giant cells may be responsible for in vivo production of proMMP-3 by the stromal cells. The authors propose that GCT has a self-stimulatory system for the production of matrix-degrading proteinases and that the ability of the passaged stromal cells to synthesize and secrete proMMP-3 with appropriate stimuli may contribute the malignant behavior of GCT.
Full text
PDF

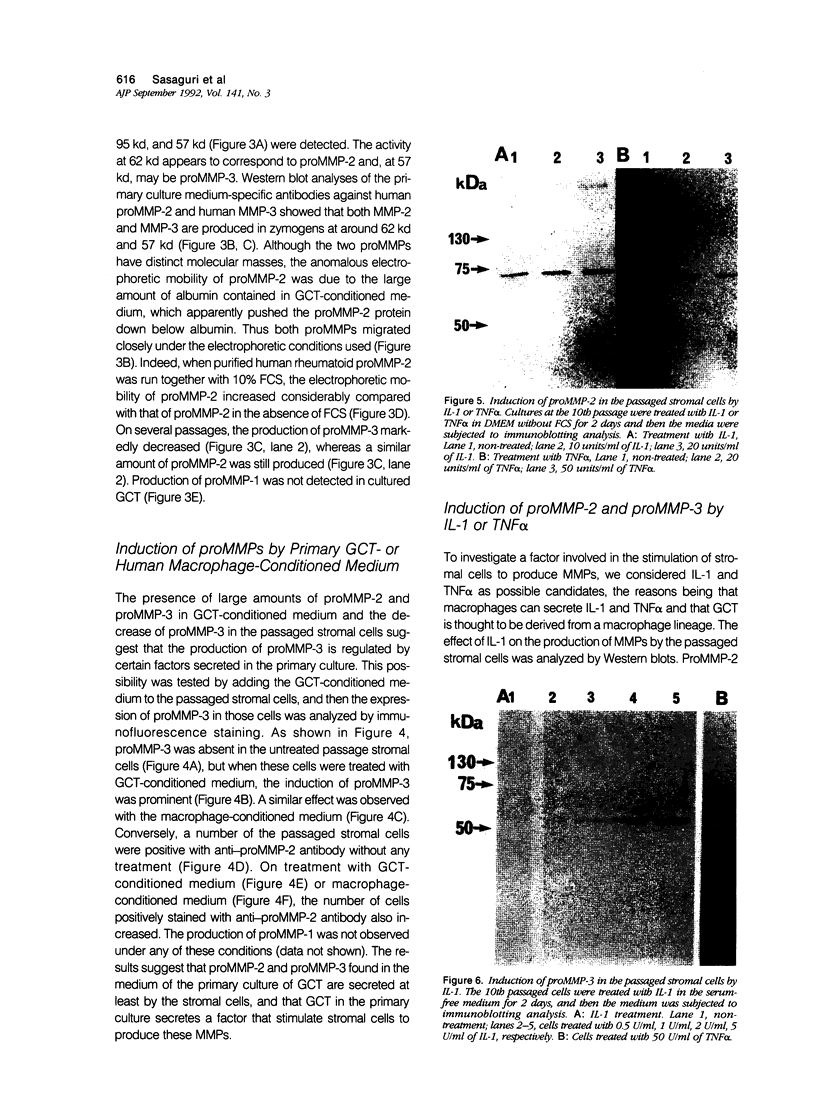

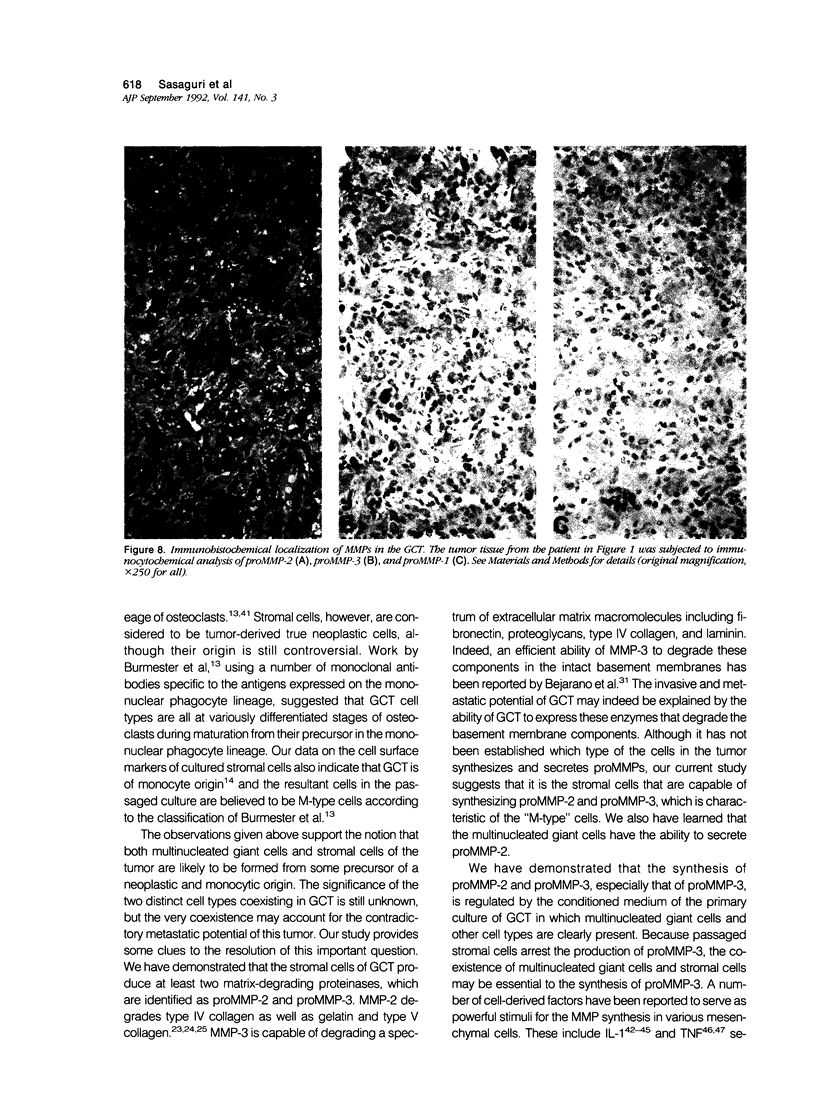



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer E. A., Cooper T. W., Huang J. S., Altman J., Deuel T. F. Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4132–4136. doi: 10.1073/pnas.82.12.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano P. A., Noelken M. E., Suzuki K., Hudson B. G., Nagase H. Degradation of basement membranes by human matrix metalloproteinase 3 (stromelysin). Biochem J. 1988 Dec 1;256(2):413–419. doi: 10.1042/bj2560413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley-Sturrock A., Woodward S. C., Senior R. M., Griffin G. L., Klagsbrun M., Davidson J. M. Differential stimulation of collagenase and chemotactic activity in fibroblasts derived from rat wound repair tissue and human skin by growth factors. J Cell Physiol. 1989 Jan;138(1):70–78. doi: 10.1002/jcp.1041380111. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Winchester R. J., Dimitriu-Bona A., Klein M., Steiner G., Sissons H. A. Delineation of four cell types comprising the giant cell tumor of bone. Expression of Ia and monocyte-macrophage lineage antigens. J Clin Invest. 1983 Jun;71(6):1633–1648. doi: 10.1172/JCI110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Separation of lymphocytes, granulocytes, and monocytes from human blood using iodinated density gradient media. Methods Enzymol. 1984;108:88–102. doi: 10.1016/s0076-6879(84)08076-9. [DOI] [PubMed] [Google Scholar]
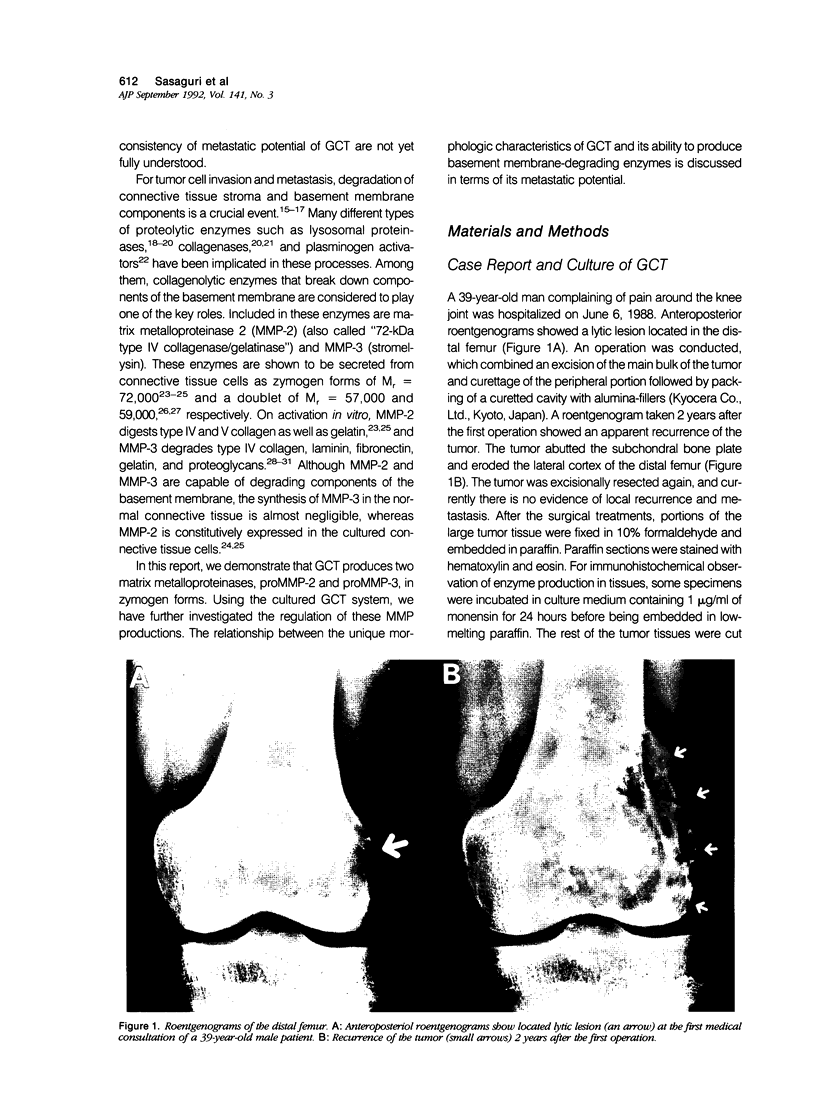
- Caballes R. L. The mechanism of metastasis in the so-called "benign giant cell tumor of bone". Hum Pathol. 1981 Aug;12(8):762–767. doi: 10.1016/s0046-8177(81)80182-7. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Dahlin D. C., Cupps R. E., Johnson E. W., Jr Giant-cell tumor: a study of 195 cases. Cancer. 1970 May;25(5):1061–1070. doi: 10.1002/1097-0142(197005)25:5<1061::aid-cncr2820250509>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Bréard J., Chess L., Krane S. M. Participation of monocyte-macrophages and lymphocytes in the production of a factor that stimulates collagenase and prostaglandin release by rheumatoid synovial cells. J Clin Invest. 1979 Nov;64(5):1386–1392. doi: 10.1172/JCI109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Zavadil-Grob C., Ucla C., Mach B. Induction of human interleukin 1 mRNA measured by collagenase- and prostaglandin E2-stimulating activity in rheumatoid synovial cells. Eur J Immunol. 1984 Oct;14(10):898–901. doi: 10.1002/eji.1830141007. [DOI] [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg R. R., Campbell C. J., Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970 Jun;52(4):619–664. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., Meats J. E., Russell R. G. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984 Feb 14;797(2):186–193. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Ito A., Nagase H. Evidence that human rheumatoid synovial matrix metalloproteinase 3 is an endogenous activator of procollagenase. Arch Biochem Biophys. 1988 Nov 15;267(1):211–216. doi: 10.1016/0003-9861(88)90025-2. [DOI] [PubMed] [Google Scholar]
- JEWELL J. H., BUSH L. F. "BENIGN" GIANT-CELL TUMOR OF BONE WITH A SOLITARY PULMONARY METASTASIS. A CASE REPORT. J Bone Joint Surg Am. 1964 Jun;46:848–852. [PubMed] [Google Scholar]
- Komiya S., Inoue A., Nakashima M., Ueno A., Fujikawa K., Ikuta H. Prognostic factors in giant cell tumor of bone. A modified histological grading system useful as a guide to prognosis. Arch Orthop Trauma Surg. 1986;105(2):67–72. doi: 10.1007/BF00455841. [DOI] [PubMed] [Google Scholar]
- Komiya S., Sasaguri Y., Inoue A., Nakashima M., Yamamoto S., Yanagida I., Morimatsu M. Characterization of cells cultured from human giant-cell tumors of bone. Phenotypic relationship to the monocyte-macrophage and osteoclast. Clin Orthop Relat Res. 1990 Sep;(258):304–309. [PubMed] [Google Scholar]
- Lin C. W., Phillips S. L., Brinckerhoff C. E., Georgescu H. I., Bandara G., Evans C. H. Induction of collagenase mRNA in lapine articular chondrocytes by synovial factors and interleukin-1. Arch Biochem Biophys. 1988 Jul;264(1):351–354. doi: 10.1016/0003-9861(88)90605-4. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A., Thorgeirsson U. P., Garbisa S. Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1982;1(4):277–288. doi: 10.1007/BF00124213. [DOI] [PubMed] [Google Scholar]
- MURPHY W. R., ACKERMAN L. V. Benign and malignant giant-cell tumors of bone; a clinical-pathological evaluation of thirty-one cases. Cancer. 1956 Mar-Apr;9(2):317–339. doi: 10.1002/1097-0142(195603/04)9:2<317::aid-cncr2820090220>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Tsuboi R., Robbins E., Rifkin D. B. In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol. 1989 Feb;108(2):671–682. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Kitagawa H., Aikawa Y. Tumor necrosis factor stimulates gelatinase and collagenase production by granulation tissue in culture. Biochem Biophys Res Commun. 1987 Feb 13;142(3):791–797. doi: 10.1016/0006-291x(87)91483-5. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Okada Y., Harris E. D., Jr, Nagase H. The precursor of a metalloendopeptidase from human rheumatoid synovial fibroblasts. Purification and mechanisms of activation by endopeptidases and 4-aminophenylmercuric acetate. Biochem J. 1988 Sep 15;254(3):731–741. doi: 10.1042/bj2540731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Morodomi T., Enghild J. J., Suzuki K., Yasui A., Nakanishi I., Salvesen G., Nagase H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990 Dec 27;194(3):721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Okada Y., Takeuchi N., Tomita K., Nakanishi I., Nagase H. Immunolocalization of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989 Aug;48(8):645–653. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Tiltman K. J., Recklies A. D., Stoker T. A. Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomas. Nature. 1978 Jun 15;273(5663):545–547. doi: 10.1038/273545a0. [DOI] [PubMed] [Google Scholar]
- Rock M. G., Pritchard D. J., Unni K. K. Metastases from histologically benign giant-cell tumor of bone. J Bone Joint Surg Am. 1984 Feb;66(2):269–274. [PubMed] [Google Scholar]
- Sasaguri Y., Sanford T., Aguirre P., Padmanabhan R. Immunological analysis of 140-kDa adenovirus-encoded DNA polymerase in adenovirus type 2-infected HeLa cells using antibodies raised against the protein expressed in Escherichia coli. Virology. 1987 Oct;160(2):389–399. doi: 10.1016/0042-6822(87)90010-9. [DOI] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Sloane B. F., Dunn J. R., Honn K. V. Lysosomal cathepsin B: correlation with metastatic potential. Science. 1981 Jun 5;212(4499):1151–1153. doi: 10.1126/science.7233209. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V. Cysteine proteinases and metastasis. Cancer Metastasis Rev. 1984;3(3):249–263. doi: 10.1007/BF00048388. [DOI] [PubMed] [Google Scholar]
- Sung H. W., Kuo D. P., Shu W. P., Chai Y. B., Liu C. C., Li S. M. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am. 1982 Jun;64(5):755–761. [PubMed] [Google Scholar]
- Suzuki K., Enghild J. J., Morodomi T., Salvesen G., Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry. 1990 Nov 6;29(44):10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Ito A., Nagino M., Mori Y., Xie B., Nagase H. Cyclic adenosine 3',5'-monophosphate suppresses interleukin 1-induced synthesis of matrix metalloproteinases but not of tissue inhibitor of metalloproteinases in human uterine cervical fibroblasts. J Biol Chem. 1991 Oct 25;266(30):19894–19899. [PubMed] [Google Scholar]
- Triglia T., Burns G. F. A method for in vitro clearance of mycoplasma from human cell lines. J Immunol Methods. 1983 Nov 11;64(1-2):133–139. doi: 10.1016/0022-1759(83)90391-5. [DOI] [PubMed] [Google Scholar]
- Warshawsky H., Goltzman D., Rouleau M. F., Bergeron J. J. Direct in vivo demonstration by radioautography of specific binding sites for calcitonin in skeletal and renal tissues of the rat. J Cell Biol. 1980 Jun;85(3):682–694. doi: 10.1083/jcb.85.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. W., Neff J. R., Gollahon K. A., Gourley W. K. Macrophages in giant cell tumours of bone. J Pathol. 1978 May;125(1):53–58. doi: 10.1002/path.1711250108. [DOI] [PubMed] [Google Scholar]
- Woolley D. E. Collagenolytic mechanisms in tumor cell invasion. Cancer Metastasis Rev. 1984;3(4):361–372. doi: 10.1007/BF00051460. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]