Abstract
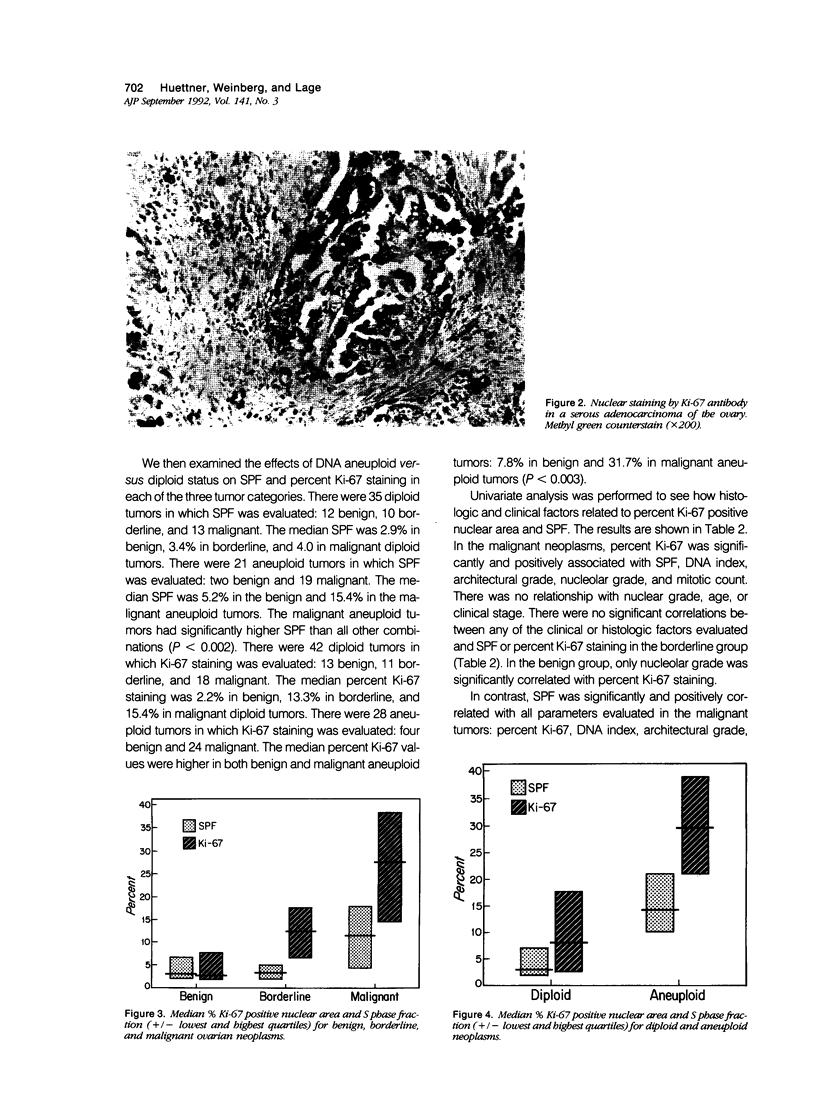
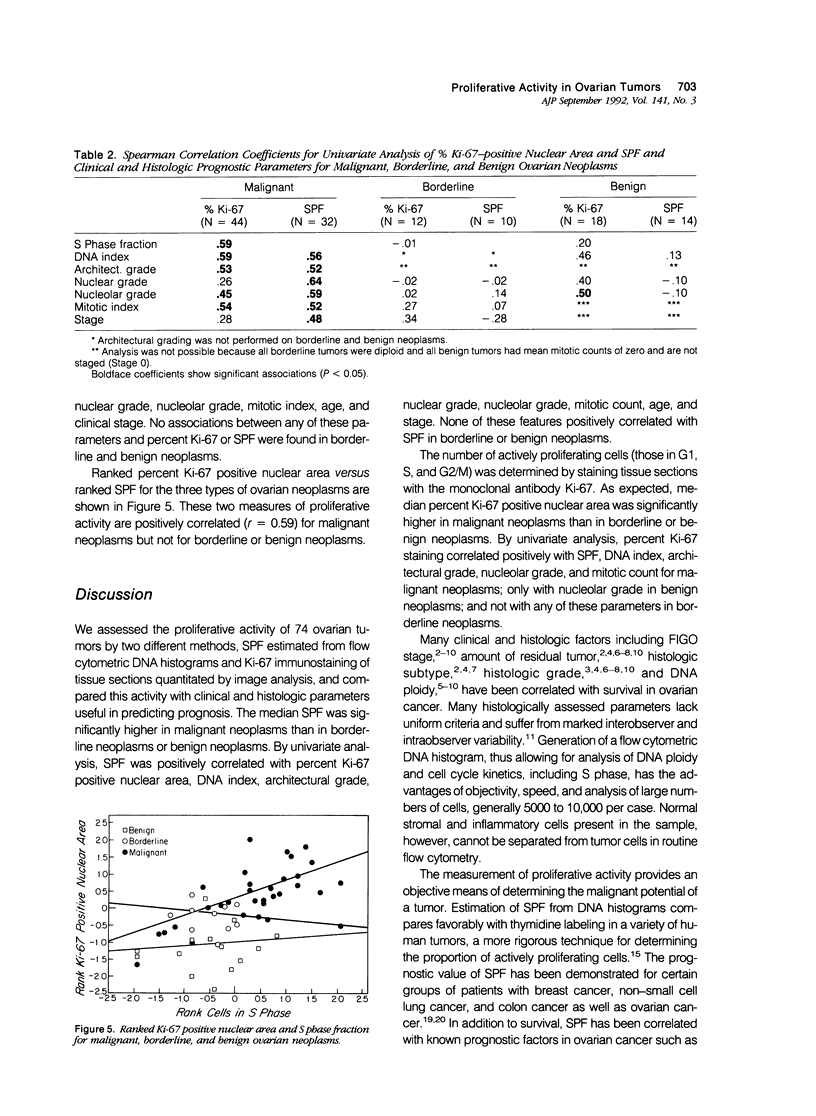
We undertook a prospective flow and static cytometric study of proliferative activity in 74 malignant, borderline and benign ovarian neoplasms. Proliferative activity as assessed by S phase fraction (SPF), and immunostaining of tissue sections with Ki-67 antibody was compared with prognostically important clinicopathologic features. Malignant neoplasms had higher median percentage Ki-67 staining (27.6%) than borderline (12.3%) and benign (2.7%) tumors (P less than 0.05). Percentage Ki-67 staining correlated with SPF, DNA index, architectural grade, nucleolar grade, and mitotic count in malignant tumors; with nucleolar grade in benign tumors and with none of these variables in borderline tumors. Similarly, malignant neoplasms had a higher median SPF (11.5%) than borderline (3.4%) and benign (2.9%) neoplasms (P less than 0.05). In malignant neoplasms, SPF correlated with percentage Ki-67 staining, DNA index, age, and stage, but with none of these in borderline or benign neoplasms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aure J. C., Hoeg K., Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971 Jan;37(1):1–9. [PubMed] [Google Scholar]
- Baak J. P., Langley F. A., Talerman A., Delemarre J. F. Interpathologist and intrapathologist disagreement in ovarian tumor grading and typing. Anal Quant Cytol Histol. 1986 Dec;8(4):354–357. [PubMed] [Google Scholar]
- Baisch H., Göhde W., Linden W. A. Analysis of PCP-data to determine the fraction of cells in the various phases of cell cycle. Radiat Environ Biophys. 1975 Jun 13;12(1):31–39. doi: 10.1007/BF02339807. [DOI] [PubMed] [Google Scholar]
- Barnard N. J., Hall P. A., Lemoine N. R., Kadar N. Proliferative index in breast carcinoma determined in situ by Ki67 immunostaining and its relationship to clinical and pathological variables. J Pathol. 1987 Aug;152(4):287–295. doi: 10.1002/path.1711520407. [DOI] [PubMed] [Google Scholar]
- Brescia R. J., Barakat R. A., Beller U., Frederickson G., Suhrland M. J., Dubin N., Demopoulos R. I. The prognostic significance of nuclear DNA content in malignant epithelial tumors of the ovary. Cancer. 1990 Jan 1;65(1):141–147. doi: 10.1002/1097-0142(19900101)65:1<141::aid-cncr2820650128>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Cole D., Gatter K. C., Mason D. Y. Carcinoma of the cervix uteri: an assessment of tumour proliferation using the monoclonal antibody Ki67. Br J Cancer. 1988 Feb;57(2):178–181. doi: 10.1038/bjc.1988.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. E., Norton J. A., Weinberg D. S. Comparative assessment of proliferation and DNA content in breast carcinoma by image analysis and flow cytometry. Am J Pathol. 1990 May;136(5):1115–1124. [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Fletcher J. A., Gibas Z., Donovan K., Perez-Atayde A., Genest D., Morton C. C., Lage J. M. Ovarian granulosa-stromal cell tumors are characterized by trisomy 12. Am J Pathol. 1991 Mar;138(3):515–520. [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Swanson C., Russell P. Prediction of long-term survival by flow cytometric analysis of cellular DNA content in patients with advanced ovarian cancer. J Clin Oncol. 1988 Feb;6(2):282–290. doi: 10.1200/JCO.1988.6.2.282. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Taylor I. W., Russell P., Coates A. S., Tattersall M. H. Influence of cellular DNA content on survival in advanced ovarian cancer. Cancer Res. 1984 Jan;44(1):397–400. [PubMed] [Google Scholar]
- Friedlander M. L., Russell P., Taylor I. W., Hedley D. W., Tattersall M. H. Flow cytometric analysis of cellular DNA content as an adjunct to the diagnosis of ovarian tumours of borderline malignancy. Pathology. 1984 Jul;16(3):301–306. doi: 10.3109/00313028409068541. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Taylor I. W., Russell P., Musgrove E. A., Hedley D. H., Tattersall M. H. Ploidy as a prognostic factor in ovarian cancer. Int J Gynecol Pathol. 1983;2(1):55–63. doi: 10.1097/00004347-198301000-00005. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lelle R. J., Pickartz H., Heidenreich W., Schwarting R., Kurtsiefer L., Stauch G., Stein H. Growth fractions in breast cancers determined in situ with monoclonal antibody Ki-67. J Clin Pathol. 1986 Sep;39(9):977–980. doi: 10.1136/jcp.39.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Richards M. A., Gregory W. M., d'Ardenne A. J., Lister T. A., Stansfeld A. G. The prognostic value of Ki67 immunostaining in non-Hodgkin's lymphoma. J Pathol. 1988 Mar;154(3):223–235. doi: 10.1002/path.1711540305. [DOI] [PubMed] [Google Scholar]
- Hiddemann W., Schumann J., Andreef M., Barlogie B., Herman C. J., Leif R. C., Mayall B. H., Murphy R. F., Sandberg A. A. Convention on nomenclature for DNA cytometry. Committee on Nomenclature, Society for Analytical Cytology. Cancer Genet Cytogenet. 1984 Oct;13(2):181–183. doi: 10.1016/0165-4608(84)90059-1. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Jo S., Akuta K., Nishimura Y., Takahashi M., Abe M. Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer. 1987 Jul 1;60(1):121–127. doi: 10.1002/1097-0142(19870701)60:1<121::aid-cncr2820600123>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Isola J., Kallioniemi O. P., Korte J. M., Wahlström T., Aine R., Helle M., Helin H. Steroid receptors and Ki-67 reactivity in ovarian cancer and in normal ovary: correlation with DNA flow cytometry, biochemical receptor assay, and patient survival. J Pathol. 1990 Dec;162(4):295–301. doi: 10.1002/path.1711620404. [DOI] [PubMed] [Google Scholar]
- Iversen O. E., Skaarland E. Ploidy assessment of benign and malignant ovarian tumors by flow cytometry. A clinicopathologic study. Cancer. 1987 Jul 1;60(1):82–87. doi: 10.1002/1097-0142(19870701)60:1<82::aid-cncr2820600114>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Mattila J., Punnonen R., Koivula T. DNA ploidy level and cell cycle distribution in ovarian cancer: relation to histopathological features of the tumor. Int J Gynecol Pathol. 1988;7(1):1–11. doi: 10.1097/00004347-198803000-00001. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Punnonen R., Mattila J., Lehtinen M., Koivula T. Prognostic significance of DNA index, multiploidy, and S-phase fraction in ovarian cancer. Cancer. 1988 Jan 15;61(2):334–339. doi: 10.1002/1097-0142(19880115)61:2<334::aid-cncr2820610224>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kurtin P. J., Pinkus G. S. Leukocyte common antigen--a diagnostic discriminant between hematopoietic and nonhematopoietic neoplasms in paraffin sections using monoclonal antibodies: correlation with immunologic studies and ultrastructural localization. Hum Pathol. 1985 Apr;16(4):353–365. doi: 10.1016/s0046-8177(85)80229-x. [DOI] [PubMed] [Google Scholar]
- Lage J. M., Driscoll S. G., Yavner D. L., Olivier A. P., Mark S. D., Weinberg D. S. Hydatidiform moles. Application of flow cytometry in diagnosis. Am J Clin Pathol. 1988 May;89(5):596–600. doi: 10.1093/ajcp/89.5.596. [DOI] [PubMed] [Google Scholar]
- Lage J. M., Weinberg D. S., Huettner P. C., Mark S. D. Flow cytometric analysis of nuclear DNA content in ovarian tumors. Association of ploidy with tumor type, histologic grade, and clinical stage. Cancer. 1992 Jun 1;69(11):2668–2675. doi: 10.1002/1097-0142(19920601)69:11<2668::aid-cncr2820691108>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- McGurrin J. F., Doria M. I., Jr, Dawson P. J., Karrison T., Stein H. O., Franklin W. A. Assessment of tumor cell kinetics by immunohistochemistry in carcinoma of breast. Cancer. 1987 May 15;59(10):1744–1750. doi: 10.1002/1097-0142(19870515)59:10<1744::aid-cncr2820591012>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Merkel D. E., McGuire W. L. Ploidy, proliferative activity and prognosis. DNA flow cytometry of solid tumors. Cancer. 1990 Mar 1;65(5):1194–1205. doi: 10.1002/1097-0142(19900301)65:5<1194::aid-cncr2820650528>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Coplin M. D. Thymidine labeling index, flow cytometric S-phase measurement, and DNA index in human tumors. Comparisons and correlations. Am J Clin Pathol. 1988 May;89(5):586–595. doi: 10.1093/ajcp/89.5.586. [DOI] [PubMed] [Google Scholar]
- Nishizaki T., Orita T., Furutani Y., Ikeyama Y., Aoki H., Sasaki K. Flow-cytometric DNA analysis and immunohistochemical measurement of Ki-67 and BUdR labeling indices in human brain tumors. J Neurosurg. 1989 Mar;70(3):379–384. doi: 10.3171/jns.1989.70.3.0379. [DOI] [PubMed] [Google Scholar]
- Rodenburg C. J., Cornelisse C. J., Heintz P. A., Hermans J., Fleuren G. J. Tumor ploidy as a major prognostic factor in advanced ovarian cancer. Cancer. 1987 Jan 15;59(2):317–323. doi: 10.1002/1097-0142(19870115)59:2<317::aid-cncr2820590225>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Sahin A. A., Ro J. Y., el-Naggar A. K., Wilson P. L., Teague K., Blick M., Ayala A. G. Tumor proliferative fraction in solid malignant neoplasms. A comparative study of Ki-67 immunostaining and flow cytometric determinations. Am J Clin Pathol. 1991 Oct;96(4):512–519. doi: 10.1093/ajcp/96.4.512. [DOI] [PubMed] [Google Scholar]
- Schwartz B. R., Pinkus G., Bacus S., Toder M., Weinberg D. S. Cell proliferation in non-Hodgkin's lymphomas. Digital image analysis of Ki-67 antibody staining. Am J Pathol. 1989 Feb;134(2):327–336. [PMC free article] [PubMed] [Google Scholar]
- Seckinger D., Sugarbaker E., Frankfurt O. DNA content in human cancer. Application in pathology and clinical medicine. Arch Pathol Lab Med. 1989 Jun;113(6):619–626. [PubMed] [Google Scholar]
- Shepherd N. A., Richman P. I., England J. Ki-67 derived proliferative activity in colorectal adenocarcinoma with prognostic correlations. J Pathol. 1988 Jul;155(3):213–219. doi: 10.1002/path.1711550306. [DOI] [PubMed] [Google Scholar]
- Sigurdsson K., Alm P., Gullberg B. Prognostic factors in malignant epithelial ovarian tumors. Gynecol Oncol. 1983 Jun;15(3):370–380. doi: 10.1016/0090-8258(83)90055-0. [DOI] [PubMed] [Google Scholar]
- Sorbe B., Frankendal B., Veress B. Importance of histologic grading in the prognosis of epithelial ovarian carcinoma. Obstet Gynecol. 1982 May;59(5):576–582. [PubMed] [Google Scholar]
- Wong W. S., Tattersall M. H. Immunohistochemical determination of tumour growth fraction in human ovarian carcinoma. Br J Obstet Gynaecol. 1989 Jun;96(6):720–724. doi: 10.1111/j.1471-0528.1989.tb03289.x. [DOI] [PubMed] [Google Scholar]
- Zehr R. J., Bauer T. W., Marks K. E., Weltevreden A. Ki-67 and grading of malignant fibrous histiocytomas. Cancer. 1990 Nov 1;66(9):1984–1990. doi: 10.1002/1097-0142(19901101)66:9<1984::aid-cncr2820660923>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Zuber P., Hamou M. F., de Tribolet N. Identification of proliferating cells in human gliomas using the monoclonal antibody Ki-67. Neurosurgery. 1988 Feb;22(2):364–368. doi: 10.1227/00006123-198802000-00015. [DOI] [PubMed] [Google Scholar]