Abstract
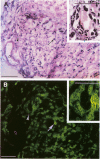
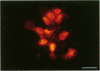
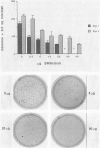
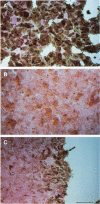
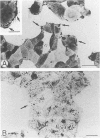
BR96 is a monoclonal antibody (MAb) that recognizes many human carcinomas and can kill antigen-positive tumor cells in vitro. Using both gold and radiolabeled MAb, the distribution and cellular processing of BR96 during cytolysis has been determined. After a brief (< 3 minutes) MAb treatment, cells in suspension are stained by the nuclear viability dye propidium iodide. Whole MAb and F(ab')2 fragments are equally cytotoxic; monovalent F(ab) fragments, however, have no effect on dye uptake unless cross-linked with goat anti-mouse IgG. The level of toxicity is dependent on both MAb dose and on cell surface receptor density. Cell contact may regulate receptor expression. BR96 receptors are more abundant on cells migrating into the open areas of a scratch wounded confluent culture than on the adjacent contact-inhibited cells. BR96 can also inhibit the anchorage-independent growth of tumor cells in soft agar showing that its effects on propidium iodide staining are not due to transient changes in membrane permeability. Immunogold electron microscopy reveals that, after a 1-minute treatment, BR96 induces significant infolding of the plasma membrane and that internalized MAb is localized to these structures. Immediately thereafter, large cell surface and intracellular vesicles form, mitochondria are swollen, and membrane integrity is lost. Therefore, BR96 seems to cause morphological changes characteristic of necrosis rather than apoptosis. When bound to adherent carcinoma cells, BR96 is distributed uniformly on the apical surface of cells labeled at 4 C and is enriched at points of cell substratum contact. Upon warming of the cells to 37 C, BR96 localizes in small perinuclear clusters and the cell margin is now devoid of label. Immunogold electron microscopy reveals that BR96 undergoes receptor mediated internalization and is localized within the same coated pits, endosomes, and lysosomes as the transferrin receptor. Quantitative studies using iodinated BR96 show that after 6 hours of chase, a maximum of 53% of the radiolabel is located within the intracellular pool. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis indicates that 84% of this fraction is nondegraded. BR96 probably cycles between the medium and intracellular pools because the remainder of the radiolabel is in the medium as intact MAb. By 24 hours of chase, the intracellular fraction drops to 30%, while the remaining 70% is present in the culture medium, mostly as low molecular weight degradation products.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danscher G. Localization of gold in biological tissue. A photochemical method for light and electronmicroscopy. Histochemistry. 1981;71(1):81–88. doi: 10.1007/BF00592572. [DOI] [PubMed] [Google Scholar]
- Drebin J. A., Link V. C., Stern D. F., Weinberg R. A., Greene M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985 Jul;41(3):697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- Espevik T., Brockhaus M., Loetscher H., Nonstad U., Shalaby R. Characterization of binding and biological effects of monoclonal antibodies against a human tumor necrosis factor receptor. J Exp Med. 1990 Feb 1;171(2):415–426. doi: 10.1084/jem.171.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström I., Garrigues H. J., Garrigues U., Hellström K. E. Highly tumor-reactive, internalizing, mouse monoclonal antibodies to Le(y)-related cell surface antigens. Cancer Res. 1990 Apr 1;50(7):2183–2190. [PubMed] [Google Scholar]
- Hopkins C. R., Gibson A., Shipman M., Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990 Jul 26;346(6282):335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Köhler H. R., Dhein J., Alberti G., Krammer P. H. Ultrastructural analysis of apoptosis induced by the monoclonal antibody anti-APO-1 on a lymphoblastoid B cell line. Ultrastruct Pathol. 1990 Nov-Dec;14(6):513–518. doi: 10.3109/01913129009076138. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987 Jun 5;49(5):669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Masui H., Kawamoto T., Sato J. D., Wolf B., Sato G., Mendelsohn J. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1984 Mar;44(3):1002–1007. [PubMed] [Google Scholar]
- Miyawaki T., Yachie A., Uwadana N., Ohzeki S., Nagaoki T., Taniguchi N. Functional significance of Tac antigen expressed on activated human T lymphocytes: Tac antigen interacts with T cell growth factor in cellular proliferation. J Immunol. 1982 Dec;129(6):2474–2478. [PubMed] [Google Scholar]
- Oi V. T., Glazer A. N., Stryer L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol. 1982 Jun;93(3):981–986. doi: 10.1083/jcb.93.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren R., Takahashi S., Doss C., Levy R., Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990 Aug;10(8):4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosok M. J., Stebbins M. R., Connelly K., Lostrom M. E., Siadak A. W. Generation and characterization of murine antiflagellum monoclonal antibodies that are protective against lethal challenge with Pseudomonas aeruginosa. Infect Immun. 1990 Dec;58(12):3819–3828. doi: 10.1128/iai.58.12.3819-3828.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal A., Hakomori S. Molecular changes in carbohydrate antigens associated with cancer. Bioessays. 1990 May;12(5):223–230. doi: 10.1002/bies.950120506. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Taylor D. S., Nowell P. C., Kornbluth J. Functional role of HLA class I cell-surface molecules in human T-lymphocyte activation and proliferation. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4446–4450. doi: 10.1073/pnas.83.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trail P. A., Willner D., Lasch S. J., Henderson A. J., Greenfield R. S., King D., Zoeckler M. E., Braslawsky G. R. Antigen-specific activity of carcinoma-reactive BR64-doxorubicin conjugates evaluated in vitro and in human tumor xenograft models. Cancer Res. 1992 Oct 15;52(20):5693–5700. [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Domingo D. L. Anti-transferrin receptor monoclonal antibody and toxin-antibody conjugates affect growth of human tumour cells. Nature. 1981 Nov 12;294(5837):171–173. doi: 10.1038/294171a0. [DOI] [PubMed] [Google Scholar]
- Walker N. I., Harmon B. V., Gobé G. C., Kerr J. F. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yeh C. J., Hsi B. L., Faulk W. P. Propidium iodide as a nuclear marker in immunofluorescence. II. Use with cellular identification and viability studies. J Immunol Methods. 1981;43(3):269–275. doi: 10.1016/0022-1759(81)90174-5. [DOI] [PubMed] [Google Scholar]