Abstract
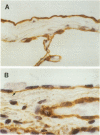
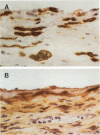
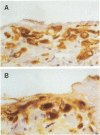
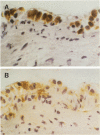
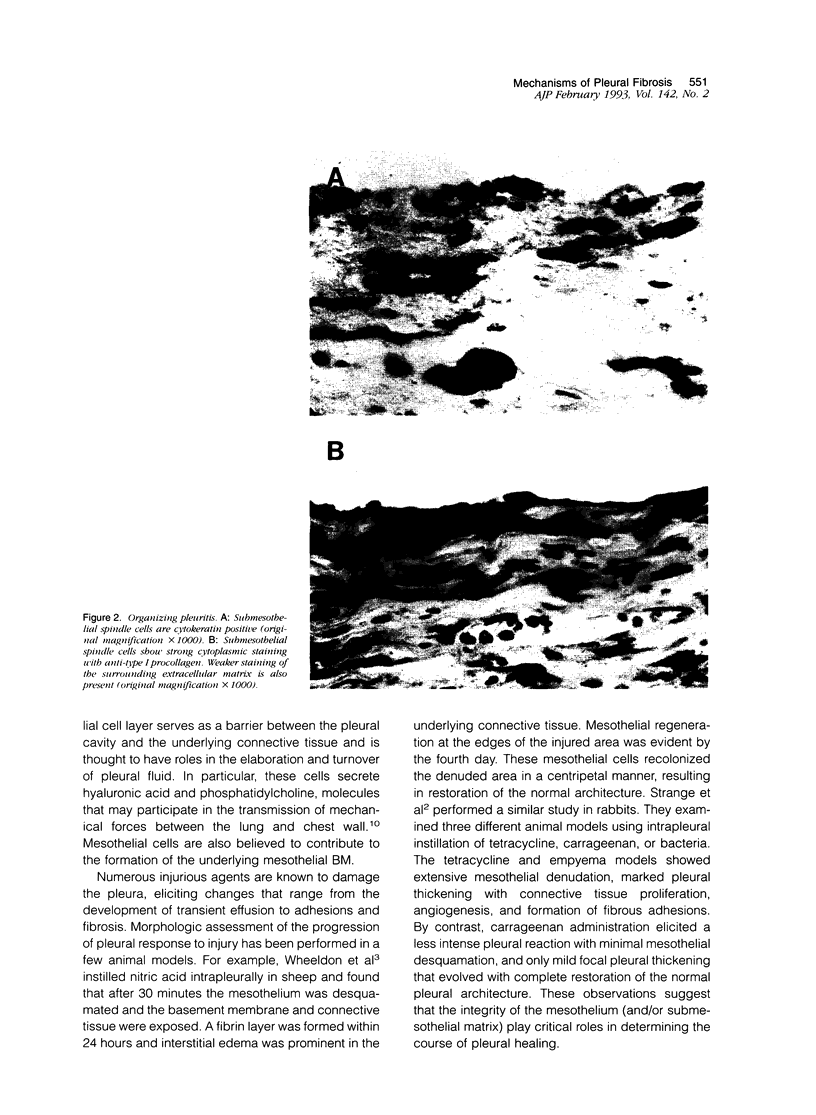
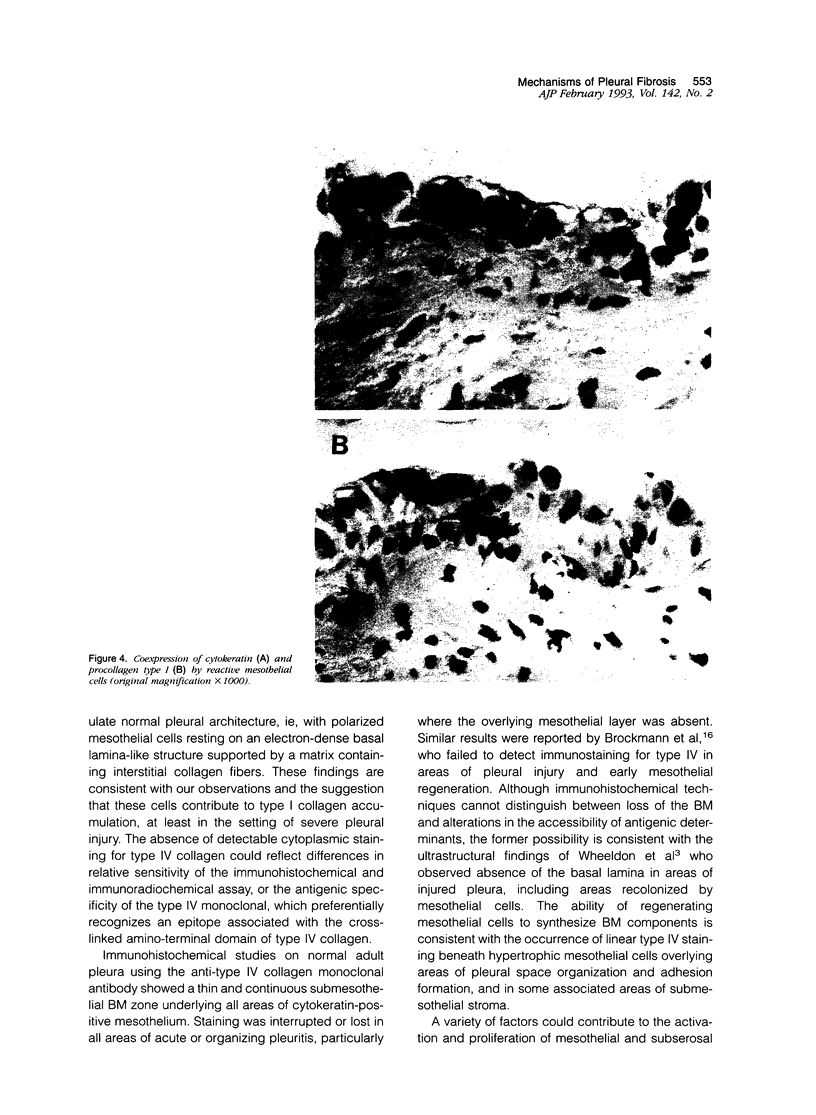
The pleural response to injury is a complex and poorly understood multifactorial process that can result in the development of fibrosis or obliteration of the pleural space. Pleural fibroblasts are considered the main source of extracellular matrix but cell culture studies have demonstrated synthesis of matrix components by mesothelial cells. We assessed the mesothelial cell contribution to extracellular matrix during pleural healing using immunohistochemical technique. Paraffin-embedded tissue of 3 normal adult lungs and 7 adults with active pleuritis were studied using monoclonal antibodies to cytokeratin, type IV collagen, vimentin, and type I procollagen (PCI). Normal pleural had a single layer of cytokeratin-positive and PCI-negative mesothelium over a thin, continuous type IV collagen-positive basement membrane and PCI-negative submesothelial stroma. Areas of active pleuritis showed loss of the continuous linear staining with anti-type IV collagen antibody. Coexpression of cytokeratin, vimentin and PCI was identified in spindle and/or cuboidal cells located in the fibrin layer, submesothelial connective tissue layer, or on the pleural surface. These findings suggest that reactive mesothelial cells play an active role in the production of extracellular matrix during pleural injury, and that disruption of the submesothelial basement membrane is a key event in determining subsequent fibrous organization of pleural exudate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. W., Hammar S. P., McNutt M. A. Reactive and neoplastic serosal tissue. A light-microscopic, ultrastructural, and immunocytochemical study. Am J Surg Pathol. 1986 Jan;10(1):34–47. doi: 10.1097/00000478-198601000-00005. [DOI] [PubMed] [Google Scholar]
- Brockmann M., Brockmann I., Fischer M., Müller K. M. Reactive lesions of the pleura. Immunohistochemical characterization. Pathol Res Pract. 1990 Apr;186(2):238–246. doi: 10.1016/S0344-0338(11)80541-8. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., Welgus H. G. Extracellular matrix injury during lung inflammation. Chest. 1987 Jul;92(1):161–167. doi: 10.1378/chest.92.1.161. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Greenberg J. Modulation of the effects of alveolar macrophages on lung fibroblast collagen production rate. Am Rev Respir Dis. 1987 Jan;135(1):52–56. doi: 10.1164/arrd.1987.135.1.52. [DOI] [PubMed] [Google Scholar]
- Dobbie J. W., Pavlina T., Lloyd J., Johnson R. C. Phosphatidylcholine synthesis by peritoneal mesothelium: its implications for peritoneal dialysis. Am J Kidney Dis. 1988 Jul;12(1):31–36. doi: 10.1016/s0272-6386(88)80068-4. [DOI] [PubMed] [Google Scholar]
- Harvey W., Amlot P. L. Collagen production by human mesothelial cells in vitro. J Pathol. 1983 Mar;139(3):337–347. doi: 10.1002/path.1711390309. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Boldt J., King T. E., Jr, Crouch E., Vartio T., McDonald J. A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989 Dec;140(6):1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- Kuwahara M., Kuwahara M., Bijwaard K. E., Gersten D. M., Diglio C. A., Kagan E. Mesothelial cells produce a chemoattractant for lung fibroblasts: role of fibronectin. Am J Respir Cell Mol Biol. 1991 Sep;5(3):256–264. doi: 10.1165/ajrcmb/5.3.256. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Matheke M. L., Crouch E., Koo M., Kuhn C., 3rd A monoclonal antibody to the carboxyterminal domain of procollagen type I visualizes collagen-synthesizing fibroblasts. Detection of an altered fibroblast phenotype in lungs of patients with pulmonary fibrosis. J Clin Invest. 1986 Nov;78(5):1237–1244. doi: 10.1172/JCI112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery A. T. Regeneration of parietal and visceral peritoneum. A light microscopical study. Br J Surg. 1973 Apr;60(4):293–299. doi: 10.1002/bjs.1800600412. [DOI] [PubMed] [Google Scholar]
- Raftery A. T. Regeneration of parietal and visceral peritoneum: an electron microscopical study. J Anat. 1973 Sep;115(Pt 3):375–392. [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Jaurand M. C., Bignon J., Kawanami O., Ferrans V. J., Davidson J., Crystal R. G. Role of pleural mesothelial cells in the production of the submesothelial connective tissue matrix of lung. Am Rev Respir Dis. 1984 Aug;130(2):267–274. doi: 10.1164/arrd.1984.130.2.267. [DOI] [PubMed] [Google Scholar]
- Strange C., Tomlinson J. R., Wilson C., Harley R., Miller K. S., Sahn S. A. The histology of experimental pleural injury with tetracycline, empyema, and carrageenan. Exp Mol Pathol. 1989 Dec;51(3):205–219. doi: 10.1016/0014-4800(89)90020-8. [DOI] [PubMed] [Google Scholar]
- Stylianou E., Jenner L. A., Davies M., Coles G. A., Williams J. D. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990 Jun;37(6):1563–1570. doi: 10.1038/ki.1990.150. [DOI] [PubMed] [Google Scholar]
- Tani K., Yasuoka S., Ogushi F., Asada K., Fujisawa K., Ozaki T., Sano N., Ogura T. Thrombin enhances lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991 Jul;5(1):34–40. doi: 10.1165/ajrcmb/5.1.34. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Wang N. S. The regional difference of pleural mesothelial cells in rabbits. Am Rev Respir Dis. 1974 Nov;110(5):623–633. doi: 10.1164/arrd.1974.110.5.623. [DOI] [PubMed] [Google Scholar]
- Whitaker D., Papadimitriou J. Mesothelial healing: morphological and kinetic investigations. J Pathol. 1985 Feb;145(2):159–175. doi: 10.1002/path.1711450204. [DOI] [PubMed] [Google Scholar]