Abstract
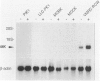
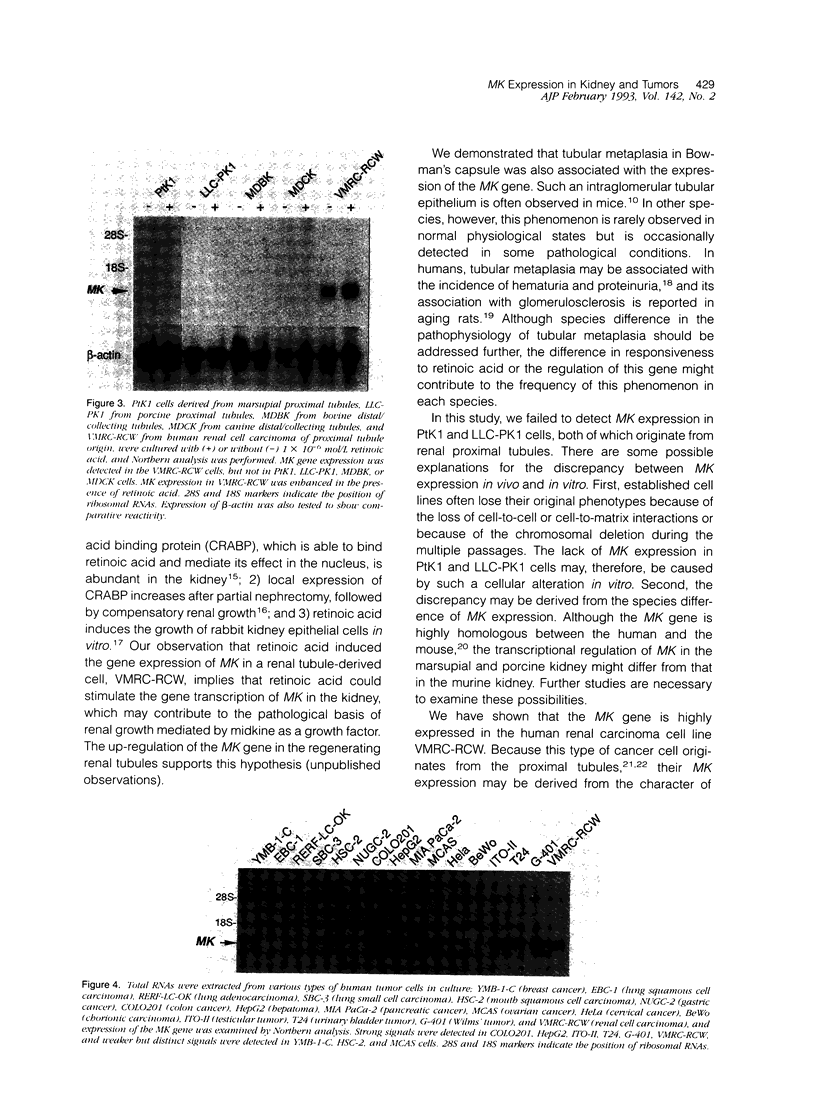
The aim of this study was to survey the expression of an embryonic cytokine gene, MK, in the normal organs and neoplastic tissues of adults. Northern analysis showed that MK mRNA was exclusively expressed in the kidney among murine organs including thymus, lung, heart, spleen, liver, and kidney. In situ hybridization analysis revealed that MK expression was localized in the proximal tubules and metaplastic Bowman's epithelium, but not in other nephron segments such as glomeruli, loop of Henle, distal tubules, and collecting ducts. To investigate whether MK expression is a marker of tubular cell lineage, several cell lines originating from renal tubules were tested. No expression of MK was detected in PtK1 and LLC-PK1 cells derived from marsupial and porcine proximal tubules or in MDBK and MDCK cells from bovine and canine distal/collecting tubules. Unexpectedly, the MK gene was expressed in a human renal cell carcinoma line, VMRC-RCW, and the expression was up-regulated in the presence of retinoic acid. To elucidate the involvement of MK in the development of tumors, we further examined its expression in a variety of human neoplastic cell lines: YMB-1-C (breast cancer), EBC-1 (lung squamous cell carcinoma), RERF-LC-OK (lung adenocarcinoma), SBC-3 (lung small cell carcinoma), HSC-2 (mouth squamous cell carcinoma), NUGC-2 (gastric cancer), COLO201 (colon cancer), HepG2 (hepatoma), MIA PaCa-2 (pancreatic cancer), MCAS (ovarian cancer), HeLa (cervical cancer), BeWo (chorionic carcinoma), ITO-II (testicular tumor), T24 (urinary bladder tumor), and G-401 (Wilms' tumor). Strong signals were detected in COLO201, HepG2, ITO-II, T24, G-401, and weaker but distinct signals were detected in YMB-1-C, HSC-2, and MCAS cells. The MK gene was, therefore, widely expressed in neoplastic cells originating from genital organs, intestinal tract, liver, mammary gland, and urinary tract, and the expression was not restricted to adenocarcinomas, but was also observed in other types of tumor cells. These findings suggest that a retinoic acid responsive gene, MK, may play a role in the pathophysiology of renal proximal tubules and tumorigenesis in many types of neoplasms.
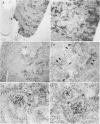
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argiles A., Mourad G., Basset N., Axelrud-Cavadore C., Haiech J., Mion C., Cavadore J. C., Demaille J. G. Acute adaptative changes to unilateral nephrectomy in humans. Kidney Int. 1987 Nov;32(5):714–720. doi: 10.1038/ki.1987.265. [DOI] [PubMed] [Google Scholar]
- Argilés A., Kraft N. E., Hutchinson P., Senes-Ferrari S., Atkins R. C. Retinoic acid affects the cell cycle and increases total protein content in epithelial cells. Kidney Int. 1989 Dec;36(6):954–959. doi: 10.1038/ki.1989.287. [DOI] [PubMed] [Google Scholar]
- Castelletto L., Goya R. G. Sex-related incidence of tubular metaplasia in Bowman's capsule of aging rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(2):79–82. doi: 10.1007/BF02899390. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Paasivuo R., Lehto V. P., Linder E., Alfthan O., Virtanen I. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest. 1983 Sep;49(3):317–326. [PubMed] [Google Scholar]
- Kadomatsu K., Huang R. P., Suganuma T., Murata F., Muramatsu T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J Cell Biol. 1990 Mar;110(3):607–616. doi: 10.1083/jcb.110.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadomatsu K., Tomomura M., Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1312–1318. doi: 10.1016/s0006-291x(88)80505-9. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Kitamura A., Mitarai T., Maruyama N., Nagasawa R., Kawamura T., Yoshida H., Takahashi T., Sakai O. Gene expression of metalloproteinase and its inhibitor in mesangial cells exposed to high glucose. Biochem Biophys Res Commun. 1992 Jun 30;185(3):1048–1054. doi: 10.1016/0006-291x(92)91732-6. [DOI] [PubMed] [Google Scholar]
- Muramatsu H., Muramatsu T. Purification of recombinant midkine and examination of its biological activities: functional comparison of new heparin binding factors. Biochem Biophys Res Commun. 1991 Jun 14;177(2):652–658. doi: 10.1016/0006-291x(91)91838-4. [DOI] [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong D. E., Crow J. A., Chytil F. Radioimmunochemical determination of cellular retinol- and cellular retinoic acid-binding proteins in cytosols of rat tissues. J Biol Chem. 1982 Nov 25;257(22):13385–13389. [PubMed] [Google Scholar]
- Segal R., Fine L. G. Polypeptide growth factors and the kidney. Kidney Int Suppl. 1989 Nov;27:S2–10. [PubMed] [Google Scholar]
- Tomomura M., Kadomatsu K., Nakamoto M., Muramatsu H., Kondoh H., Imagawa K., Muramatsu T. A retinoic acid responsive gene, MK, produces a secreted protein with heparin binding activity. Biochem Biophys Res Commun. 1990 Sep 14;171(2):603–609. doi: 10.1016/0006-291x(90)91189-y. [DOI] [PubMed] [Google Scholar]
- Valdes A. J., Zhang J. M. Intraglomerular tubular epithelial cells. A marker of glomerular hematuria. Arch Pathol Lab Med. 1987 Feb;111(2):189–191. [PubMed] [Google Scholar]
- Wallace A. C., Nairn R. C. Renal tubular antigens in kidney tumors. Cancer. 1972 Apr;29(4):977–981. doi: 10.1002/1097-0142(197204)29:4<977::aid-cncr2820290444>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Sherwood A. C. Tubulocystic epithelium. Kidney Int. 1991 Mar;39(3):450–463. doi: 10.1038/ki.1991.56. [DOI] [PubMed] [Google Scholar]
- Wolf G., Neilson E. G. Molecular mechanisms of tubulointerstitial hypertrophy and hyperplasia. Kidney Int. 1991 Mar;39(3):401–420. doi: 10.1038/ki.1991.52. [DOI] [PubMed] [Google Scholar]