Abstract
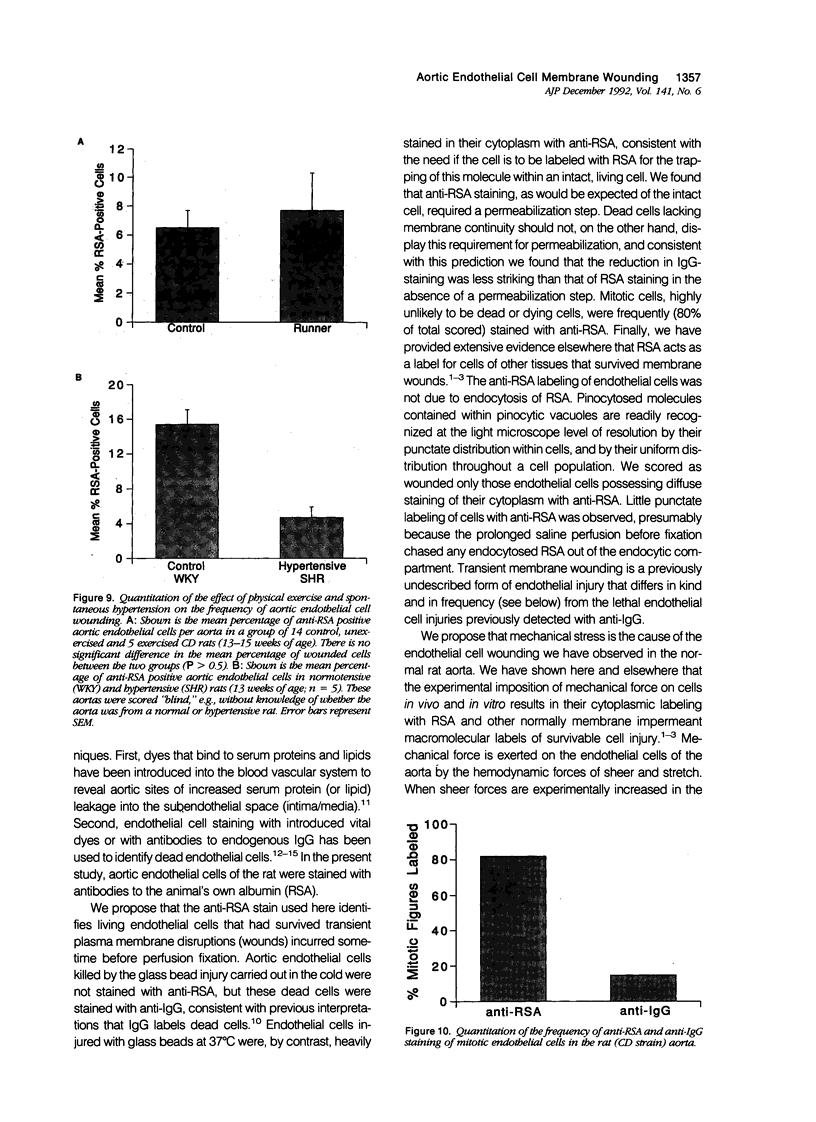
Cells of gut, skin, and muscle frequently suffer transient survivable plasma membrane disruptions ("wounds") under physiological conditions, but it is not known whether endothelial cells of the aorta, which are constantly exposed to hemodynamically generated mechanical forces, similarly are injured in vivo. We have used serum albumin as a molecular probe for identifying endothelial cells of the rat aorta that incurred and survived transient plasma membrane wounds in vivo. Such wounded endothelial cells were in fact observed in the aortas of all rats examined. However, the percentage of wounded cells in the total aortic endothelial population varied remarkably between individuals ranging from 1.4% to 17.9% with a mean of 6.5% (+/- 4.6% SD). Wounded endothelial cells were heterogeneously distributed, being found in distinct clusters often in the shape of streaks aligned with the long axis of the vessel, or in the shape of partial or complete rims surrounding bifurcation openings, such as the ostia of the intercostal arteries. Physical exercise (running) did not increase the frequency of aortic endothelial cell membrane wounding, nor did spontaneous hypertension. Surprisingly, 80% of mitotic endothelial cell figures were identified as wounded. This article identified a previously unrecognized form of endothelial cell injury, survivable disruptions of the plasma membrane, and shows that injury to the endothelial cells of the normal aorta is far more commonplace than previously suspected. Plasma membrane wounding of endothelial cells could be linked to the initiation of atherosclerosis.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Björkerud S., Bondjers G. Endothelial integrity and viability in the aorta of the normal rabbit and rat as evaluated with dye exclusion tests and interference contrast microscopy. Atherosclerosis. 1972 May-Jun;15(3):285–300. doi: 10.1016/0021-9150(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Fry D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968 Feb;22(2):165–197. doi: 10.1161/01.res.22.2.165. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Goode T. B., Davies P. F., Reidy M. A., Bowyer D. E. Aortic endothelial cell morphology observed in situ by scanning electron microscopy during atherogenesis in the rabbit. Atherosclerosis. 1977 Jun;27(2):235–251. doi: 10.1016/0021-9150(77)90061-2. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Bondjers G., Bylock A., Hjalmarsson L. Ultrastructural studies on the localization of IgG in the aortic endothelium and subendothelial intima of atherosclerotic and nonatherosclerotic rabbits. Exp Mol Pathol. 1980 Dec;33(3):302–315. doi: 10.1016/0014-4800(80)90028-3. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Chao S., Schwartz S. M., Reidy M. A. Aortic endothelial cell death and replication in normal and lipopolysaccharide-treated rats. Am J Pathol. 1985 Oct;121(1):123–127. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Schwartz S. M. Evidence for cell death in the vascular endothelium in vivo and in vitro. Am J Pathol. 1983 Sep;112(3):278–286. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Zand T., Majno G. Hydrodynamic injury of the endothelium in acute aortic stenosis. Am J Pathol. 1982 Mar;106(3):394–408. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Edelman E. R. Biological and biochemical properties of fibroblast growth factors. Implications for the pathogenesis of atherosclerosis. Arteriosclerosis. 1989 May-Jun;9(3):269–278. doi: 10.1161/01.atv.9.3.269. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. The affinity of fibroblast growth factors (FGFs) for heparin; FGF-heparan sulfate interactions in cells and extracellular matrix. Curr Opin Cell Biol. 1990 Oct;2(5):857–863. doi: 10.1016/0955-0674(90)90084-r. [DOI] [PubMed] [Google Scholar]
- Lin S. J., Jan K. M., Chien S. Role of dying endothelial cells in transendothelial macromolecular transport. Arteriosclerosis. 1990 Sep-Oct;10(5):703–709. doi: 10.1161/01.atv.10.5.703. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989 May;96(5 Pt 1):1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J Cell Sci. 1990 Jul;96(Pt 3):549–556. doi: 10.1242/jcs.96.3.549. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Muthukrishnan L., Warder E., D'Amore P. A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989 Aug;109(2):811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Warder E. Glass beads load macromolecules into living cells. J Cell Sci. 1987 Dec;88(Pt 5):669–678. doi: 10.1242/jcs.88.5.669. [DOI] [PubMed] [Google Scholar]
- Nilsson J. Growth factors and the pathogenesis of atherosclerosis. Atherosclerosis. 1986 Dec;62(3):185–199. doi: 10.1016/0021-9150(86)90093-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Aortic endothelial cell replication. I. Effects of age and hypertension in the rat. Circ Res. 1977 Aug;41(2):248–255. doi: 10.1161/01.res.41.2.248. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]









