Abstract
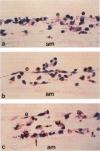
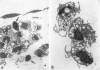
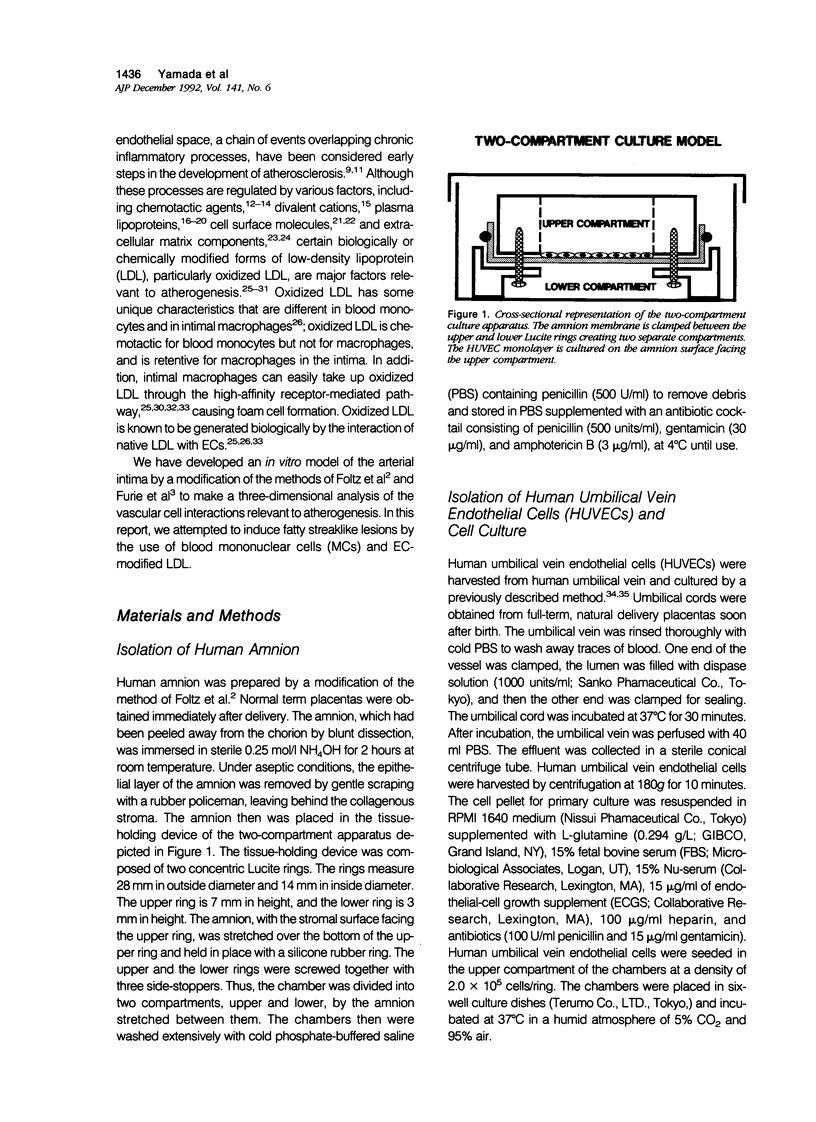
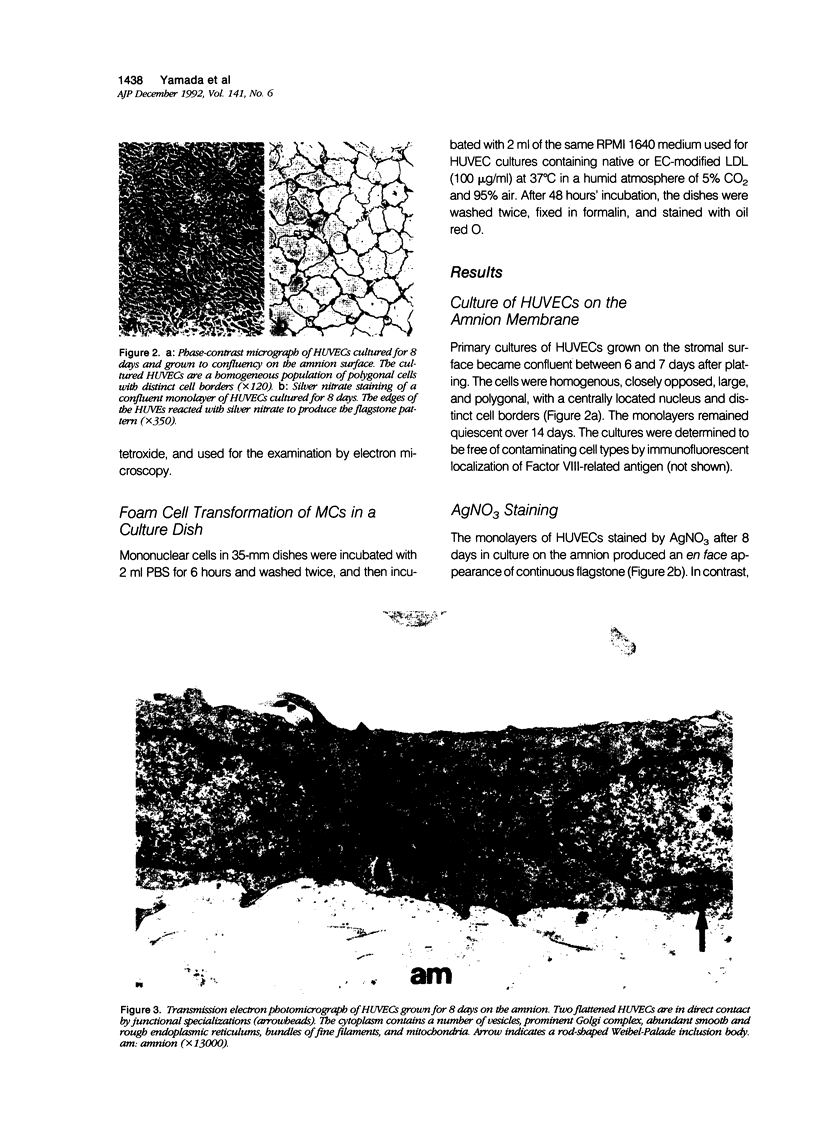
In this study a two-compartment culture model of arterial intima was used for the in vitro induction of fatty streaklike lesions. The apparatus consisted of upper and lower compartments separated by a human amnion membrane stretched between them. Human umbilical vein endothelial cells (HUVECs) were cultured to confluence on the stromal surface of the amnion membrane. Maximal migration of blood mononuclear cells (MCs) through the HUVEC monolayer in response to a f-Met-Leu-Phe gradient was observed at 10(-8) mol/l; the migration was 3.29 times greater than that observed under the condition of random migration (control). In the study of MC transformation into lipid-laden cells in the amnion membrane (foam cell formation in 'arterial intima'), 10(6) MCs were incubated, in the presence of freshly prepared low-density lipoprotein (LDL; 100 microgram/ml). The lipid loading of MCs was time dependent. After 12 hours' incubation, 39% of the MCs that migrated into the amnion membrane contained a small number of lipid droplets, whereas the remaining 61% showed no lipid droplets. Only 1.7% of the cells contained a high number of lipid droplets in the cytoplasm and took on the appearance of foam cells. With time, the number of lipid-laden cells and the amounts of intracytoplasmic lipid droplets gradually increased. At 72 hours after incubation, 65.4% of the MCs were loaded with lipid droplets, and 20.9% of them, an eightfold increase over 12 hours of incubation, showed a foamy cell appearance. Because MCs consist of 70% monocytes and 30% lymphocytes, about 93% of the monocytes were filled with lipid after a 72-hour incubation. Ultrastructural examination showed that lipid-laden cells took on macrophage characteristics, such as wide and heterogeneous cytoplasm, indented nuclei, and abundant lysosomes. A minority of the MCs in the amnion were considered lymphocytes; they had scanty cytoplasm, round nuclei with abundant heterochromatin, no lysosomes, and no lipid vacuoles. In conclusion, the formation of an in vitro fatty streaklike lesion is demonstrated, and this is reminiscent of in vivo human atherogenesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson L. M., Endemann G., Lindsey S., Pronczuk A., Hoover R. L., Hayes K. C. LDL enhances monocyte adhesion to endothelial cells in vitro. Am J Pathol. 1986 May;123(2):334–342. [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Monocytic origin of foam cells in human atherosclerotic plaques. Atherosclerosis. 1984 Dec;53(3):265–271. doi: 10.1016/0021-9150(84)90127-8. [DOI] [PubMed] [Google Scholar]
- BOURNE G. L. The microscopic anatomy of the human amnion and chorion. Am J Obstet Gynecol. 1960 Jun;79:1070–1073. doi: 10.1016/0002-9378(60)90512-3. [DOI] [PubMed] [Google Scholar]
- Babaev V. R., Bobryshev Y. V., Stenina O. V., Tararak E. M., Gabbiani G. Heterogeneity of smooth muscle cells in atheromatous plaque of human aorta. Am J Pathol. 1990 May;136(5):1031–1042. [PMC free article] [PubMed] [Google Scholar]
- Boyd H. C., Gown A. M., Wolfbauer G., Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989 Nov;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of human blood monocytes with Nycodenz, a new non-ionic iodinated gradient medium. Scand J Immunol. 1983 May;17(5):429–436. doi: 10.1111/j.1365-3083.1983.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Denholm E. M., Lewis J. C. Monocyte chemoattractants in pigeon aortic atherosclerosis. Am J Pathol. 1987 Mar;126(3):464–475. [PMC free article] [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Endemann G., Pronzcuk A., Friedman G., Lindsey S., Alderson L., Hayes K. C. Monocyte adherence to endothelial cells in vitro is increased by beta-VLDL. Am J Pathol. 1987 Jan;126(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Fan J. L., Yamada T., Tokunaga O., Watanabe T. Alterations in the functional characteristics of macrophages induced by hypercholesterolemia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61(1):19–27. doi: 10.1007/BF02890401. [DOI] [PubMed] [Google Scholar]
- Foltz C. M., Russo R. G., Siegal G. P., Terranova V. P., Liotta L. A. Interactions of tumor cells with whole basement membrane in the presence or absence of endothelium. Prog Clin Biol Res. 1982;89:353–371. [PubMed] [Google Scholar]
- Frostegård J., Nilsson J., Haegerstrand A., Hamsten A., Wigzell H., Gidlund M. Oxidized low density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc Natl Acad Sci U S A. 1990 Feb;87(3):904–908. doi: 10.1073/pnas.87.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klurfeld D. M. Identification of foam cells in human atherosclerotic lesions as macrophages using monoclonal antibodies. Arch Pathol Lab Med. 1985 May;109(5):445–449. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorisi G., Folkes E., Pawlowski N., Cramer E. B. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am J Pathol. 1987 Apr;127(1):157–167. [PMC free article] [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski N. A., Abraham E. L., Pontier S., Scott W. A., Cohn Z. A. Human monocyte-endothelial cell interaction in vitro. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8208–8212. doi: 10.1073/pnas.82.23.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Repin V. S., Dolgov V. V., Zaikina O. E., Novikov I. D., Antonov A. S., Nikolaeva M. A., Smirnov V. N. Heterogeneity of endothelium in human aorta. A quantitative analysis by scanning electron microscopy. Atherosclerosis. 1984 Jan;50(1):35–52. doi: 10.1016/0021-9150(84)90006-6. [DOI] [PubMed] [Google Scholar]
- Rogers K. A., Hoover R. L., Castellot J. J., Jr, Robinson J. M., Karnovsky M. J. Dietary cholesterol-induced changes in macrophage characteristics. Relationship to atherosclerosis. Am J Pathol. 1986 Nov;125(2):284–291. [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Russo R. G., Liotta L. A., Thorgeirsson U., Brundage R., Schiffmann E. Polymorphonuclear leukocyte migration through human amnion membrane. J Cell Biol. 1981 Nov;91(2 Pt 1):459–467. doi: 10.1083/jcb.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokama T., Haraoka S., Watanabe T. Immunohistochemical and ultrastructural demonstration of the lymphocyte-macrophage interaction in human aortic intima. Mod Pathol. 1991 Jan;4(1):101–107. [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo M. C., Berliner J. A., Almada L., Ramirez R., Fogelman A. M. Beta-very low density lipoprotein pretreatment of endothelial monolayers increases monocyte adhesion. Arteriosclerosis. 1989 Nov-Dec;9(6):824–828. doi: 10.1161/01.atv.9.6.824. [DOI] [PubMed] [Google Scholar]
- Tobias J. W., Bern M. M., Netland P. A., Zetter B. R. Monocyte adhesion to subendothelial components. Blood. 1987 Apr;69(4):1265–1268. [PubMed] [Google Scholar]
- Tokunaga O., Fan J. L., Watanabe T. Atherosclerosis- and age-related multinucleated variant endothelial cells in primary culture from human aorta. Am J Pathol. 1989 Dec;135(6):967–976. [PMC free article] [PubMed] [Google Scholar]
- Tokunaga O., Watanabe T. Properties of endothelial cell and smooth muscle cell cultured in ambient pressure. In Vitro Cell Dev Biol. 1987 Aug;23(8):528–534. doi: 10.1007/BF02620969. [DOI] [PubMed] [Google Scholar]
- Tokunaga O., Yamada T., Fan J. L., Watanabe T. Age-related decline in prostacyclin synthesis by human aortic endothelial cells. Qualitative and quantitative analysis. Am J Pathol. 1991 Apr;138(4):941–949. [PMC free article] [PubMed] [Google Scholar]
- Wallis W. J., Beatty P. G., Ochs H. D., Harlan J. M. Human monocyte adherence to cultured vascular endothelium: monoclonal antibody-defined mechanisms. J Immunol. 1985 Oct;135(4):2323–2330. [PubMed] [Google Scholar]
- Watanabe T., Tokunaga O., Fan J. L., Shimokama T. Atherosclerosis and macrophages. Acta Pathol Jpn. 1989 Aug;39(8):473–486. doi: 10.1111/j.1440-1827.1989.tb01513.x. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]