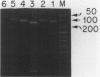
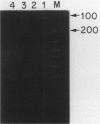
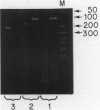
Abstract
Alternatives to Southern blot hybridization for gene rearrangement analysis are being studied because of the time, labor, cost, and radioisotopes required for this technique. We have utilized a rapid, hot air, thermocycling polymerase chain reaction (PCR) system to examine various lymphoproliferative disorders for immunoglobulin heavy chain (IgH) gene rearrangements. This unique system amplifies DNA from 10 microliters samples placed in glass capillary tubes, over a total cycle time of about 30 minutes. Amplified bands are easily visualized on ethidium bromide-stained agarose gels. Forty-one monoclonal B-cell proliferations, 27 reactive lymphoid hyperplasias, 17 T-cell lymphomas and 3 cases of Hodgkin's disease were studied. All 88 cases were fully characterized by morphologic, immunophenotypic, and genotypic (Southern blot) analyses. Each case was separately evaluated by PCR with two primer pairs: 1) IgH variable region (VH) and IgH joining region (JH) and 2) bcl-2 and JH. Thirty-four of 41 monoclonal B-cell proliferations revealed a distinct band (within an expected base pair range) with 1 or both primer combinations supporting B-cell monoclonality; the other 7 cases were considered false negatives. The 47 entities without IgH gene rearrangements detectable by Southern analysis demonstrated no amplified product or a smear of amplified DNA with no distinct band. The overall specificity of PCR was 100%, and the sensitivity was 83% when directly compared with Southern blot analysis. Although its sensitivity is currently less than optimal, PCR is a rapid and practical screening method for the detection of IgH gene rearrangements. If a positive result is obtained no further analysis is required; however, if there is a negative result, standard Southern blot analysis should be performed to definitively exclude the presence of a monoclonal B-cell population in the sample.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Wright J. J., Graninger W., Seto M., Owens J., Cossman J., Jensen J. P., Goldman P., Korsmeyer S. J. Mechanism of the t(14;18) chromosomal translocation: structural analysis of both derivative 14 and 18 reciprocal partners. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2396–2400. doi: 10.1073/pnas.84.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Alt F. W. Molecular characterization of the lymphoid V(D)J recombination activity. J Biol Chem. 1989 Jun 25;264(18):10327–10330. [PubMed] [Google Scholar]
- Brisco M. J., Tan L. W., Orsborn A. M., Morley A. A. Development of a highly sensitive assay, based on the polymerase chain reaction, for rare B-lymphocyte clones in a polyclonal population. Br J Haematol. 1990 Jun;75(2):163–167. doi: 10.1111/j.1365-2141.1990.tb02643.x. [DOI] [PubMed] [Google Scholar]
- Deane M., McCarthy K. P., Wiedemann L. M., Norton J. D. An improved method for detection of B-lymphoid clonality by polymerase chain reaction. Leukemia. 1991 Aug;5(8):726–730. [PubMed] [Google Scholar]
- Erikson J., Finan J., Tsujimoto Y., Nowell P. C., Croce C. M. The chromosome 14 breakpoint in neoplastic B cells with the t(11;14) translocation involves the immunoglobulin heavy chain locus. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4144–4148. doi: 10.1073/pnas.81.13.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Humphries C. G., Shen A., Kuziel W. A., Capra J. D., Blattner F. R., Tucker P. W. A new human immunoglobulin VH family preferentially rearranged in immature B-cell tumours. Nature. 1988 Feb 4;331(6155):446–449. doi: 10.1038/331446a0. [DOI] [PubMed] [Google Scholar]
- Kodaira M., Kinashi T., Umemura I., Matsuda F., Noma T., Ono Y., Honjo T. Organization and evolution of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1986 Aug 20;190(4):529–541. doi: 10.1016/0022-2836(86)90239-1. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Matsuda F., Kinashi T., Kodaira M., Honjo T. A novel family of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1987 Jun 20;195(4):761–768. doi: 10.1016/0022-2836(87)90482-7. [DOI] [PubMed] [Google Scholar]
- Ling F. C., Clarke C. E., Lillicrap D. Positive immunoglobulin gene rearrangement study by the polymerase chain reaction in a colonic adenocarcinoma. Am J Clin Pathol. 1992 Jul;98(1):116–119. doi: 10.1093/ajcp/98.1.116. [DOI] [PubMed] [Google Scholar]
- McCarthy K. P., Sloane J. P., Wiedemann L. M. Rapid method for distinguishing clonal from polyclonal B cell populations in surgical biopsy specimens. J Clin Pathol. 1990 May;43(5):429–432. doi: 10.1136/jcp.43.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Bienz B., Ram D., Ben-Neriah Y., Cohen J. B., Zakut R., Givol D. Organization and evolution of immunoglobulin VH gene subgroups. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4405–4409. doi: 10.1073/pnas.79.14.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said J. W., Sassoon A. F., Shintaku I. P., Corcoran P., Nichols S. W. Polymerase chain reaction for bcl-2 in diagnostic lymph node biopsies. Mod Pathol. 1990 Nov;3(6):659–663. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Segal G. H., Edinger M. G., Owen M., McNealis M., Lopez P., Perkins A., Linden M. D., Fishleder A. J., Stoler M. H., Tubbs R. R. Concomitant delineation of surface Ig, B-cell differentiation antigens, and HLADR on lymphoid proliferations using three-color immunocytometry. Cytometry. 1991;12(4):350–359. doi: 10.1002/cyto.990120410. [DOI] [PubMed] [Google Scholar]
- Segal G. H., Fishleder A. J., Stoler M. H., Tubbs R. R. Frozen sections of cellular lymphoid proliferations provide adequate DNA for routine gene rearrangement analysis. Am J Clin Pathol. 1991 Sep;96(3):360–363. doi: 10.1093/ajcp/96.3.360. [DOI] [PubMed] [Google Scholar]
- Sheibani K., Tubbs R. R. Enzyme immunohistochemistry: technical aspects. Semin Diagn Pathol. 1984 Nov;1(4):235–250. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stetlet-Stevenson M., Raffeld M., Cohen P., Cossman J. Detection of occult follicular lymphoma by specific DNA amplification. Blood. 1988 Nov;72(5):1822–1825. [PubMed] [Google Scholar]
- Trainor K. J., Brisco M. J., Story C. J., Morley A. A. Monoclonality in B-lymphoproliferative disorders detected at the DNA level. Blood. 1990 Jun 1;75(11):2220–2222. [PubMed] [Google Scholar]
- Trainor K. J., Brisco M. J., Wan J. H., Neoh S., Grist S., Morley A. A. Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain reaction. Blood. 1991 Jul 1;78(1):192–196. [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Wan J. H., Sykes P. J., Orell S. R., Morley A. A. Rapid method for detecting monoclonality in B cell lymphoma in lymph node aspirates using the polymerase chain reaction. J Clin Pathol. 1992 May;45(5):420–423. doi: 10.1136/jcp.45.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., Movahed L. A., Chen Y. Y., Shin S. S., Stroup R. M., Bui N., Estess P., Bindl J. M. Detection of immunoglobulin light-chain mRNA in lymphoid tissues using a practical in situ hybridization method. Am J Pathol. 1990 Oct;137(4):979–988. [PMC free article] [PubMed] [Google Scholar]
- Williams M. E., Innes D. J., Jr, Borowitz M. J., Lovell M. A., Swerdlow S. H., Hurtubise P. E., Brynes R. K., Chan W. C., Byrne G. E., Jr, Whitcomb C. C. Immunoglobulin and T cell receptor gene rearrangements in human lymphoma and leukemia. Blood. 1987 Jan;69(1):79–86. [PubMed] [Google Scholar]
- Wittwer C. T., Fillmore G. C., Garling D. J. Minimizing the time required for DNA amplification by efficient heat transfer to small samples. Anal Biochem. 1990 May 1;186(2):328–331. doi: 10.1016/0003-2697(90)90090-v. [DOI] [PubMed] [Google Scholar]
- Wittwer C. T., Garling D. J. Rapid cycle DNA amplification: time and temperature optimization. Biotechniques. 1991 Jan;10(1):76–83. [PubMed] [Google Scholar]
- Yamada M., Hudson S., Tournay O., Bittenbender S., Shane S. S., Lange B., Tsujimoto Y., Caton A. J., Rovera G. Detection of minimal disease in hematopoietic malignancies of the B-cell lineage by using third-complementarity-determining region (CDR-III)-specific probes. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5123–5127. doi: 10.1073/pnas.86.13.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Wasserman R., Reichard B. A., Shane S., Caton A. J., Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J Exp Med. 1991 Feb 1;173(2):395–407. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong D., Voetdijk B. M., Van Ommen G. J., Kluin-Nelemans J. C., Beverstock G. C., Kluin P. M. Translocation t(14;18) in B cell lymphomas as a cause for defective immunoglobulin production. J Exp Med. 1989 Mar 1;169(3):613–624. doi: 10.1084/jem.169.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]