Abstract
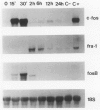
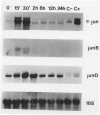
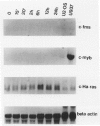
The availability of specific reagents to measure gene activity has provided important tools and potential new directions for the study of smooth muscle cell (SMC) proliferation in vivo. In this report, we have measured steady-state mRNA levels of several fos and jun family members in aortic tissue by Northern blotting after vascular injury. In addition, protein products of these genes were analyzed by immunocytochemistry. Within 15 minutes of balloon injury, mRNA levels of c-fos, fosB, c-jun, junB, and junD were elevated severalfold. In contrast, fos-related antigen (fra-1) mRNA showed a delayed onset of expression. The expression kinetics of these immediate early genes was similar to those in cultured cells stimulated to undergo proliferation by growth factors, suggesting that such SMC gene activation in vivo reflects permeation of blood-derived growth factors into the vessel wall or intravascular release of preformed growth factors. Translation of fos and jun genes into immunoreactive products was demonstrated 2 hours after balloon injury with antisera to Fos and Jun proteins. Treating rats with cycloheximide abolished this immunoreactivity. The distribution of Fos and Jun products was concentrated in SMC nuclei at the luminal border of the rat aorta. Such focal expression may have consequences for the initiation of SMC DNA synthesis and migration after vascular injury. Furthermore, the expression of Fos and Jun proteins in SMC after vascular balloon injury may be used as an index of SMC activation under a variety of experimental settings.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. C., Lam J. Y., Badimon L., Chesebro J. H., Fuster V. Interactions of platelets and vessel wall in the development of restenosis after coronary angioplasty. Ann N Y Acad Sci. 1987;516:605–620. doi: 10.1111/j.1749-6632.1987.tb33076.x. [DOI] [PubMed] [Google Scholar]
- Bravo R. Growth factor-responsive genes in fibroblasts. Cell Growth Differ. 1990 Jun;1(6):305–309. [PubMed] [Google Scholar]
- Burns E. R., Spaet T. H., Stemerman M. B. Response of the arterial wall to endothelial removal: an autoradiographic study. Proc Soc Exp Biol Med. 1978 Dec;159(3):473–477. doi: 10.3181/00379727-159-40373. [DOI] [PubMed] [Google Scholar]
- Cassis L. A., Lynch K. R., Peach M. J. Localization of angiotensinogen messenger RNA in rat aorta. Circ Res. 1988 Jun;62(6):1259–1262. doi: 10.1161/01.res.62.6.1259. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Clowes A. W., Schwartz S. M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985 Jan;56(1):139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- Cowley B. D., Jr, Chadwick L. J., Grantham J. J., Calvet J. P. Sequential protooncogene expression in regenerating kidney following acute renal injury. J Biol Chem. 1989 May 15;264(14):8389–8393. [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C., Byers M. R., Iadarola M. J., Powers E. A. Patterns of epithelial expression of Fos protein suggest important role in the transition from viable to cornified cell during keratinization. Development. 1991 Feb;111(2):253–258. doi: 10.1242/dev.111.2.253. [DOI] [PubMed] [Google Scholar]
- Friedman R. J., Stemerman M. B., Wenz B., Moore S., Gauldie J., Gent M., Tiell M. L., Spaet H. The effect of thrombocytopenia on experimental arteriosclerotic lesion formation in rabbits. Smooth muscle cell proliferation and re-endothelialization. J Clin Invest. 1977 Nov;60(5):1191–1201. doi: 10.1172/JCI108872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I. D., Stemerman M. B., Handin R. I. Vascular permeation of platelet factor 4 after endothelial injury. Science. 1980 Aug 1;209(4456):611–612. doi: 10.1126/science.6994228. [DOI] [PubMed] [Google Scholar]
- Goldberg I. D., Stemerman M. B., Schnipper L. E., Ransil B. J., Crooks G. W., Fuhro R. L. Vascular smooth muscle cell kinetics: a new assay for studying patterns of cellular proliferation in vivo. Science. 1979 Aug 31;205(4409):920–922. doi: 10.1126/science.472713. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hsu J. C., Bravo R., Taub R. Interactions among LRF-1, JunB, c-Jun, and c-Fos define a regulatory program in the G1 phase of liver regeneration. Mol Cell Biol. 1992 Oct;12(10):4654–4665. doi: 10.1128/mcb.12.10.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita N., Seki S., Sakaguchi H., Yanai A., Kuroki T., Mizoguchi Y., Kobayashi K., Monna T. Induction of proto-oncogene c-jun product in rat liver after partial hepatectomy. Gastroenterol Jpn. 1990 Aug;25(4):512–512. doi: 10.1007/BF02779347. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Angel P., Lafyatis R., Hattori K., Kim K. Y., Sporn M. B., Karin M., Roberts A. B. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990 Apr;10(4):1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., King S. B., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989 Jun;79(6):1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano J. M., Tota R. R., Vlasic N., Danishefsky K. J., Stemerman M. B. Early proto-oncogene expression in rat aortic smooth muscle cells following endothelial removal. Am J Pathol. 1990 Oct;137(4):761–765. [PMC free article] [PubMed] [Google Scholar]
- Miano J. M., Vlasic N., Tota R. R., Stemerman M. B. Smooth muscle cell immediate-early gene and growth factor activation follows vascular injury. A putative in vivo mechanism for autocrine growth. Arterioscler Thromb. 1993 Feb;13(2):211–219. doi: 10.1161/01.atv.13.2.211. [DOI] [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Hsu J. C., Melby A. E., Bravo R., Taub R. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol Cell Biol. 1991 Jan;11(1):381–390. doi: 10.1128/mcb.11.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Mugnaini E., Dahl A. L. Zinc-aldehyde fixation for light-microscopic immunocytochemistry of nervous tissues. J Histochem Cytochem. 1983 Dec;31(12):1435–1438. doi: 10.1177/31.12.6355290. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Satake M., Yamaguchi-Iwai Y., Sakai M., Muramatsu M., Ito Y. The nuclear protooncogenes c-jun and c-fos as regulators of DNA replication. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3947–3951. doi: 10.1073/pnas.88.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nathans D., Lau L. F., Christy B., Hartzell S., Nakabeppu Y., Ryder K. Genomic response to growth factors. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):893–900. doi: 10.1101/sqb.1988.053.01.102. [DOI] [PubMed] [Google Scholar]
- Okuno H., Suzuki T., Yoshida T., Hashimoto Y., Curran T., Iba H. Inhibition of jun transformation by a mutated fos gene: design of an anti-oncogene. Oncogene. 1991 Sep;6(9):1491–1497. [PubMed] [Google Scholar]
- Pertovaara L., Sistonen L., Bos T. J., Vogt P. K., Keski-Oja J., Alitalo K. Enhanced jun gene expression is an early genomic response to transforming growth factor beta stimulation. Mol Cell Biol. 1989 Mar;9(3):1255–1262. doi: 10.1128/mcb.9.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Scanlon K. J., Jiao L., Funato T., Wang W., Tone T., Rossi J. J., Kashani-Sabet M. Ribozyme-mediated cleavage of c-fos mRNA reduces gene expression of DNA synthesis enzymes and metallothionein. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10591–10595. doi: 10.1073/pnas.88.23.10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B. Thrombogenesis of the rabbit arterial plaque. An electron microscopic study. Am J Pathol. 1973 Oct;73(1):7–26. [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. jun: oncogene and transcription factor. Adv Cancer Res. 1990;55:1–35. doi: 10.1016/s0065-230x(08)60466-2. [DOI] [PubMed] [Google Scholar]
- Yang G., Koistinaho J., Zhu S., Hervonen A. Light and electron microscopic evidences of the presence of c-fos-like immunoreactivity in the rat adrenal cortex. Histochemistry. 1989;93(2):217–221. doi: 10.1007/BF00315978. [DOI] [PubMed] [Google Scholar]