Abstract
Humans chronically infected with Schistosoma mansoni most commonly present with the relatively asymptomatic intestinal form of the disease, whereas a small minority develop hepatosplenism characterized by severe hepatic disease with portal hypertension. Investigation of hypotheses describing the pathogenic mechanisms underlying the clinical forms of the human disease has been limited by the absence of an animal model that predictably develops such a spectrum of disease. We report that inbred male CBA/J mice that are chronically infected with S. mansoni develop two distinct syndromes, hypersplenomegaly syndrome (HSS) and moderate splenomegaly syndrome (MSS). Pathologically and immunologically, MSS and HSS remarkably parallel the intestinal and hepatosplenic clinical forms, respectively, in humans. HSS affects approximately 20% of these mice and consists of massive splenomegaly, ascites, thymic atrophy, severe anemia, and cachexia. The remaining majority of mice with MSS develop moderate splenomegaly only. Histopathological features of HSS include 1) relatively extensive hepatic fibrosis and granulomatous inflammation, 2) splenic congestion, 3) lymph node plasmacytosis, and 4) worms and eggs in the pulmonary vasculature. Immunologically, the idiotypes present on antisoluble egg antigen antibodies from HSS mice are distinct from those from mice with acute infections or the chronic MSS infection. These idiotypic differences are similar to those observed in patients with intestinal and hepatosplenic forms of the disease and may have regulatory importance. Investigation of the cellular and molecular events that lead to the development of MSS and HSS may advance current understanding of the pathogenesis of the clinical forms of chronic schistosomiasis in humans.
Full text
PDF



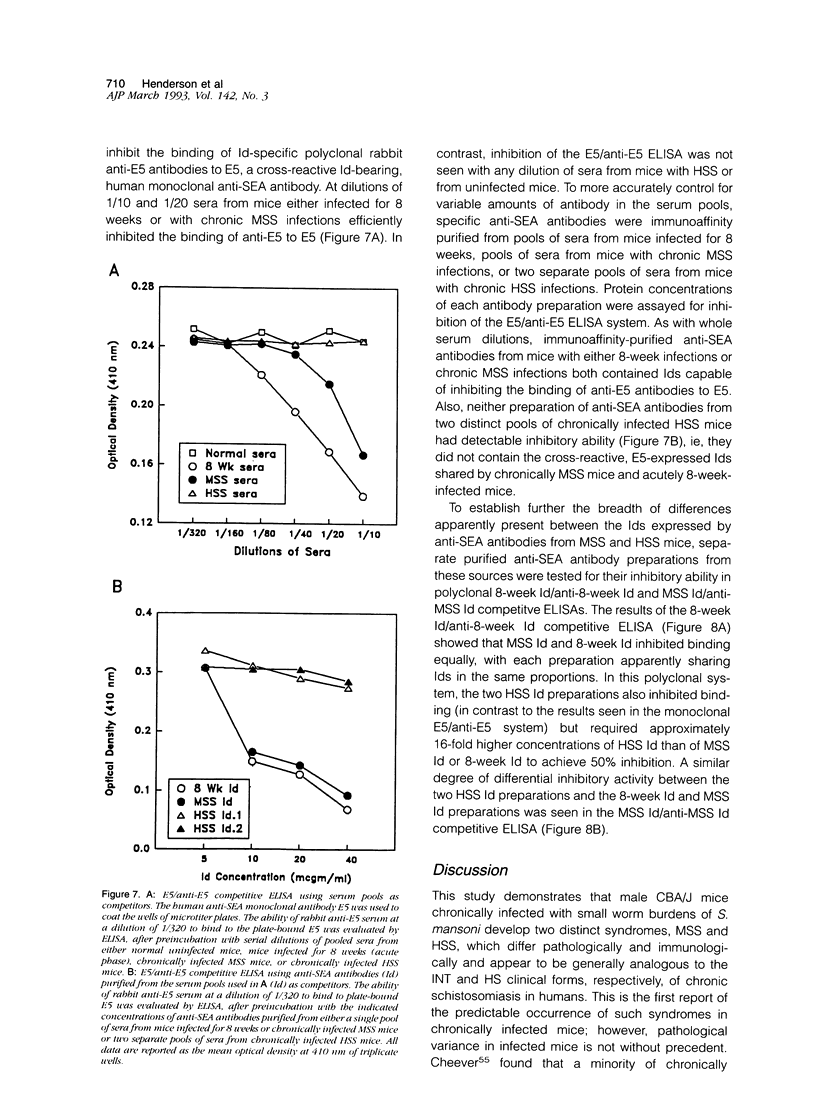
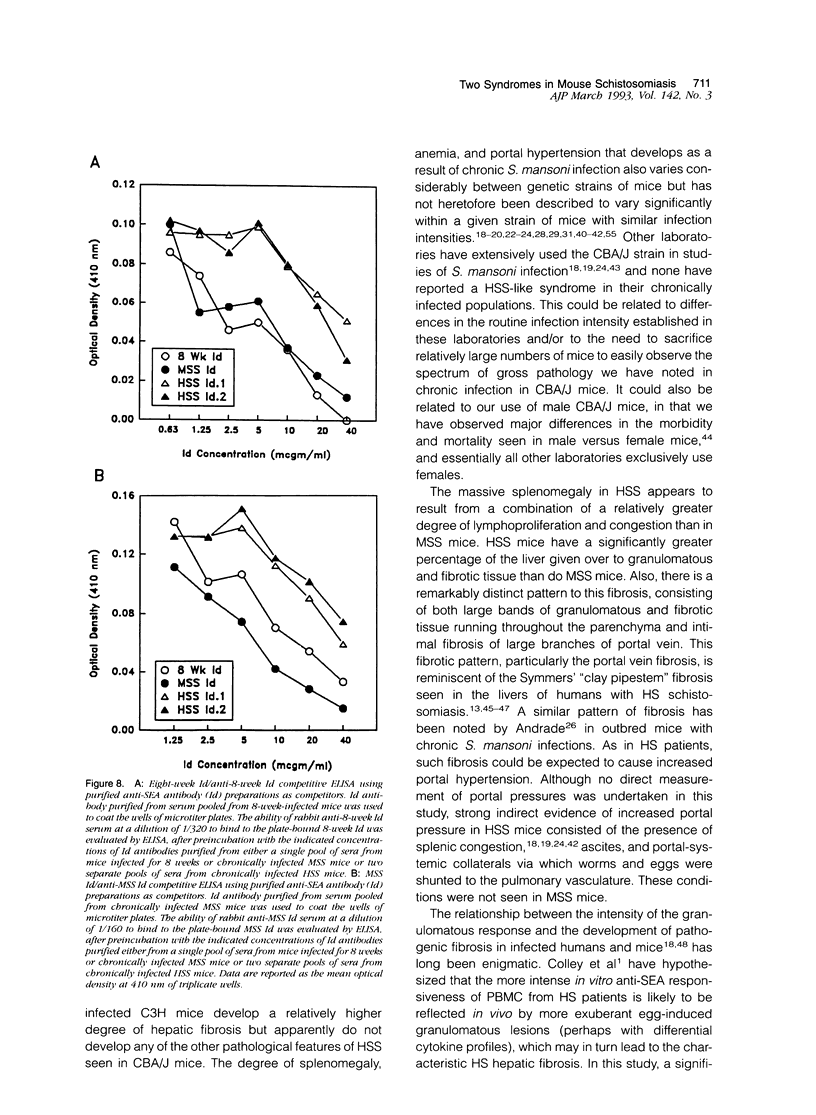



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE Z. A. WARREN KS: MILD PROLONGED SCHISTOSOMIASIS IN MICE: ALTERATIONS IN HOST RESPONSE WITH TIME AND THE DEVELOPMENT OF PORTAL FIBROSIS. Trans R Soc Trop Med Hyg. 1964 Jan;58:53–57. doi: 10.1016/0035-9203(64)90068-9. [DOI] [PubMed] [Google Scholar]
- Abdel-Salam E., Abdel Khalik A., Abdel-Meguid A., Barakat W., Mahmoud A. A. Association of HLA class I antigens (A1, B5, B8 and CW2) with disease manifestations and infection in human schistosomiasis mansoni in Egypt. Tissue Antigens. 1986 Mar;27(3):142–146. doi: 10.1111/j.1399-0039.1986.tb01513.x. [DOI] [PubMed] [Google Scholar]
- Amiri P., Locksley R. M., Parslow T. G., Sadick M., Rector E., Ritter D., McKerrow J. H. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992 Apr 16;356(6370):604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A. Pathogenesis of pipe-stem fibrosis of the liver (experimental observation on murine schistosomiasis). Mem Inst Oswaldo Cruz. 1987 Jul-Sep;82(3):325–334. doi: 10.1590/s0074-02761987000300003. [DOI] [PubMed] [Google Scholar]
- BOGLIOLO L. The anatomical picture of the liver in hepato-splenic schistosomiasis mansoni. Ann Trop Med Parasitol. 1957 Mar;51(1):1–14. doi: 10.1080/00034983.1957.11685789. [DOI] [PubMed] [Google Scholar]
- CHEEVER A. W. A COMPARATIVE STUDY OF SCHISTOSOMA MANSONI INFECTIONS IN MICE, GERBILS, MULTIMAMMATE RATS AND HAMSTERS. I. THE RELATION OF PORTAL HYPERTENSION TO SIZE OF HEPATIC GRANULOMAS. Am J Trop Med Hyg. 1965 Mar;14:211–226. doi: 10.4269/ajtmh.1965.14.211. [DOI] [PubMed] [Google Scholar]
- CHEEVER A. W. A COMPARATIVE STUDY OF SCHISTOSOMA MANSONI INFECTIONS IN MICE, GERBILS, MULTIMAMMATE RATS AND HAMSTERS. II. QUALITATIVE PATHOLOGICAL DIFFERENCES. Am J Trop Med Hyg. 1965 Mar;14:227–238. doi: 10.4269/ajtmh.1965.14.227. [DOI] [PubMed] [Google Scholar]
- CHEEVER A. W., WARREN K. S. HEPATIC BLOOD FLOW IN MICE WITH ACUTE HEPATO-SPLENIC SCHISTOSOMIASIS MANSONI. Trans R Soc Trop Med Hyg. 1964 Sep;58:406–412. doi: 10.1016/0035-9203(64)90086-0. [DOI] [PubMed] [Google Scholar]
- Cheever A. W. A quantitative post-mortem study of Schistosomiasis mansoni in man. Am J Trop Med Hyg. 1968 Jan;17(1):38–64. doi: 10.4269/ajtmh.1968.17.38. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Duvall R. H., Hallack T. A., Jr, Minker R. G., Malley J. D., Malley K. G. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am J Trop Med Hyg. 1987 Jul;37(1):85–97. doi: 10.4269/ajtmh.1987.37.85. [DOI] [PubMed] [Google Scholar]
- Claas F. H., Deelder A. M. H-2 linked immune response to murine experimental Schistosoma mansoni infections. J Immunogenet. 1979 Jun;6(3):167–175. doi: 10.1111/j.1744-313x.1979.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Colley D. G., Freeman G. L., Jr Differences in adult Schistosoma mansoni worm burden requirements for the establishment of resistance to reinfection in inbred mice. I. CBA/J and C57BL/6 mice. Am J Trop Med Hyg. 1980 Nov;29(6):1279–1285. doi: 10.4269/ajtmh.1980.29.1279. [DOI] [PubMed] [Google Scholar]
- Colley D. G., Freeman G. L., Jr Differences in adult Schistosoma mansoni worm burden requirements for the establishment of resistance to reinfection in inbred mice. II. C57BL/KsJ, SWR/J, SJL/J, BALB/cAnN, DBA/2N, A/J, B10.A(3R), and B10.A(5R) mice. Am J Trop Med Hyg. 1983 May;32(3):543–549. doi: 10.4269/ajtmh.1983.32.543. [DOI] [PubMed] [Google Scholar]
- DEWITT W. B., WARREN K. S. Hepato-splenic schistosomiasis in mice. Am J Trop Med Hyg. 1959 Jul;8(4):440–446. doi: 10.4269/ajtmh.1959.8.440. [DOI] [PubMed] [Google Scholar]
- Dean D. A., Bukowski M. A., Cheever A. W. Relationship between acquired resistance, portal hypertension, and lung granulomas in ten strains of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1981 Jul;30(4):806–814. doi: 10.4269/ajtmh.1981.30.806. [DOI] [PubMed] [Google Scholar]
- Doughty B. L., Goes A. M., Parra J. C., Rocha R., Cone J. C., Colley D. G., Gazzinelli G. Anti-idiotypic T cells in human schistosomiasis. Immunol Invest. 1989 Jan-May;18(1-4):373–388. doi: 10.3109/08820138909112250. [DOI] [PubMed] [Google Scholar]
- Dumont A. E., Becker F. F., Warren K. S., Martelli A. B. Regulation of spleen growth and portal pressure in hepatic shcistosomiasis. Am J Pathol. 1975 Feb;78(2):211–224. [PMC free article] [PubMed] [Google Scholar]
- Eloi-Santos S., Olsen N. J., Correa-Oliveira R., Colley D. G. Schistosoma mansoni: mortality, pathophysiology, and susceptibility differences in male and female mice. Exp Parasitol. 1992 Sep;75(2):168–175. doi: 10.1016/0014-4894(92)90176-b. [DOI] [PubMed] [Google Scholar]
- Fanning M. M., Peters P. A., Davis R. S., Kazura J. W., Mahmoud A. A. Immunopathology of murine infection with Schistosoma mansoni: relationship of genetic background to hepatosplenic disease and modulation. J Infect Dis. 1981 Aug;144(2):148–153. doi: 10.1093/infdis/144.2.148. [DOI] [PubMed] [Google Scholar]
- Gazzinelli G., Lambertucci J. R., Katz N., Rocha R. S., Lima M. S., Colley D. G. Immune responses during human Schistosomiasis mansoni. XI. Immunologic status of patients with acute infections and after treatment. J Immunol. 1985 Sep;135(3):2121–2127. [PubMed] [Google Scholar]
- Goes A. M., Rocha R. S., Gazzinelli G., Doughty B. L. Production and characterization of human monoclonal antibodies against Schistosoma mansoni. Parasite Immunol. 1989 Nov;11(6):695–711. doi: 10.1111/j.1365-3024.1989.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Harrison D. J., Carter C. E., Colley D. G. Immunoaffinity purification of Schistosoma mansoni soluble egg antigens. J Immunol. 1979 Jun;122(6):2210–2217. [PubMed] [Google Scholar]
- Henderson G. S., Lu X., McCurley T. L., Colley D. G. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. II. Quantification of IL-4 mRNA, IFN-gamma mRNA, and IL-2 mRNA levels in the granulomatous livers, mesenteric lymph nodes, and spleens during the course of modulation. J Immunol. 1992 Apr 1;148(7):2261–2269. [PubMed] [Google Scholar]
- Hirayama K., Matsushita S., Kikuchi I., Iuchi M., Ohta N., Sasazuki T. HLA-DQ is epistatic to HLA-DR in controlling the immune response to schistosomal antigen in humans. Nature. 1987 Jun 4;327(6121):426–430. doi: 10.1038/327426a0. [DOI] [PubMed] [Google Scholar]
- Lewis F. A., Wilson E. M. Strain differences in lymphocyte responses and in vitro suppressor cell induction between Schistosoma mansoni-infected C57BL/6 and CBA mice. Infect Immun. 1981 Apr;32(1):260–267. doi: 10.1128/iai.32.1.260-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M. S., Gazzinelli G., Nascimento E., Parra J. C., Montesano M. A., Colley D. G. Immune responses during human Schistosomiasis mansoni. Evidence for antiidiotypic T lymphocyte responsiveness. J Clin Invest. 1986 Oct;78(4):983–988. doi: 10.1172/JCI112689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. C., Ragheb S., Boros D. L. Recombinant IL-2 therapy reverses diminished granulomatous responsiveness in anti-L3T4-treated, Schistosoma mansoni-infected mice. J Immunol. 1990 Jun 1;144(11):4356–4361. [PubMed] [Google Scholar]
- Montesano M. A., Freeman G. L., Jr, Gazzinelli G., Colley D. G. Expression of cross-reactive, shared idiotypes on anti-SEA antibodies from humans and mice with schistosomiasis. J Immunol. 1990 Aug 1;145(3):1002–1008. [PubMed] [Google Scholar]
- Montesano M. A., Freeman G. L., Jr, Gazzinelli G., Colley D. G. Immune responses during human Schistosoma mansoni. XVII. Recognition by monoclonal anti-idiotypic antibodies of several idiotopes on a monoclonal anti-soluble schistosomal egg antigen antibody and anti-soluble schistosomal egg antigen antibodies from patients with different clinical forms of infection. J Immunol. 1990 Nov 1;145(9):3095–3099. [PubMed] [Google Scholar]
- Montesano M. A., Lima M. S., Correa-Oliveira R., Gazzinelli G., Colley D. G. Immune responses during human schistosomiasis mansoni. XVI. Idiotypic differences in antibody preparations from patients with different clinical forms of infection. J Immunol. 1989 Apr 1;142(7):2501–2506. [PubMed] [Google Scholar]
- Nash T. E., Cheever A. W., Ottesen E. A., Cook J. A. Schistosome infections in humans: perspectives and recent findings. NIH conference. Ann Intern Med. 1982 Nov;97(5):740–754. doi: 10.7326/0003-4819-97-5-740. [DOI] [PubMed] [Google Scholar]
- Olds G. R., el Meneza S., Mahmoud A. F., Kresina T. F. Differential immunoregulation of granulomatous inflammation, portal hypertension, and hepatic fibrosis in murine schistosomiasis mansoni. J Immunol. 1989 May 15;142(10):3605–3611. [PubMed] [Google Scholar]
- Parra J. C., Gazzinelli G., Goes A. M., Moyes R. B., Rocha R., Colley D. G., Doughty B. L. Granulomatous hypersensitivity to Schistosoma mansoni egg antigens in human schistosomiasis. II. In vitro granuloma modulation induced by polyclonal idiotypic antibodies. J Immunol. 1991 Dec 1;147(11):3949–3954. [PubMed] [Google Scholar]
- Parra J. C., Lima M. S., Gazzinelli G., Colley D. G. Immune responses during human schistosomiasis mansoni. XV. Anti-idiotypic T cells can recognize and respond to anti-SEA idiotypes directly. J Immunol. 1988 Apr 1;140(7):2401–2405. [PubMed] [Google Scholar]
- Percy A. J., Harn D. A. Monoclonal anti-idiotypic and anti-anti-idiotypic antibodies from mice immunized with a protective monoclonal antibody against Schistosoma mansoni. J Immunol. 1988 Apr 15;140(8):2760–2762. [PubMed] [Google Scholar]
- Powell M. R., Colley D. G. Anti-idiotypic T lymphocyte responsiveness in murine Schistosomiasis mansoni. Cell Immunol. 1987 Feb;104(2):377–385. doi: 10.1016/0008-8749(87)90039-6. [DOI] [PubMed] [Google Scholar]
- Powell M. R., Colley D. G. Demonstration of splenic auto-anti-idiotypic plaque-forming cells in mice infected with Schistosoma mansoni. J Immunol. 1985 Jun;134(6):4140–4145. [PubMed] [Google Scholar]
- Tweardy D. J., Osman G. S., el Kholy A., Ellner J. J. Failure of immunosuppressive mechanisms in human Schistosoma mansoni infection with hepatosplenomegaly. J Clin Microbiol. 1987 May;25(5):768–773. doi: 10.1128/jcm.25.5.768-773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lichtenberg F., Sadun E. H. Experimental production of bilharzial pipe-stem fibrosis in the chimpanzee. Exp Parasitol. 1968 Apr;22(2):264–278. doi: 10.1016/0014-4894(68)90102-1. [DOI] [PubMed] [Google Scholar]
- WARREN K. S. The contribution of worm burden and host response to the development of hepato-splenic schistosomiasis mansoni in mice. Am J Trop Med Hyg. 1963 Jan;12:34–39. doi: 10.4269/ajtmh.1963.12.34. [DOI] [PubMed] [Google Scholar]
- WARREN K. S. The etiology of hepato-splenic Schistosomiasis mansoni in mice. Am J Trop Med Hyg. 1961 Nov;10:870–876. doi: 10.4269/ajtmh.1961.10.870. [DOI] [PubMed] [Google Scholar]
- Warren K. S. Pathophysiology and pathogenesis of hepatosplenic schistosomiasis mansoni. Bull N Y Acad Med. 1968 Mar;44(3):280–294. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S. The pathogenesis of "clay-pipe stem cirrhosis" in mice with chronic schistosomiasis mansoni, with a note on the longevity of the schistosomes. Am J Pathol. 1966 Sep;49(3):477–489. [PMC free article] [PubMed] [Google Scholar]