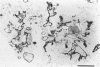Abstract
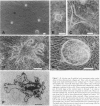
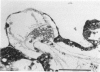
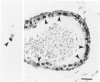
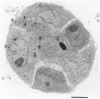
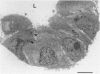
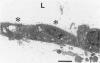
The purpose of this study is to reconstruct an alveolus-like structure from alveolar type II epithelial cells in a culture condition. Isolated alveolar type II epithelial cells of the rat were cultured in a three-dimensional collagen gel matrix. Single type II cells formed cellular aggregates that had a lumen after cell division in this culture condition. Through proliferation of the component cells, these aggregates grew to assume a globular or branching structure, part of which in turn developed into a large, cystic alveolus-like structure. This structure consisted of flattened epithelial cells intermingled with cuboidal epithelial cells. In these structures, the surfactant production was confirmed by immunohistochemistry and electron microscopy. To our knowledge, this is the first report of a reconstruction of an alveolus-like structure in a three-dimensional collagen gel matrix culture. This culture system seems to provide an appropriate physiological environment in which to study the differentiation and disorders of pulmonary alveoli.
Full text
PDF

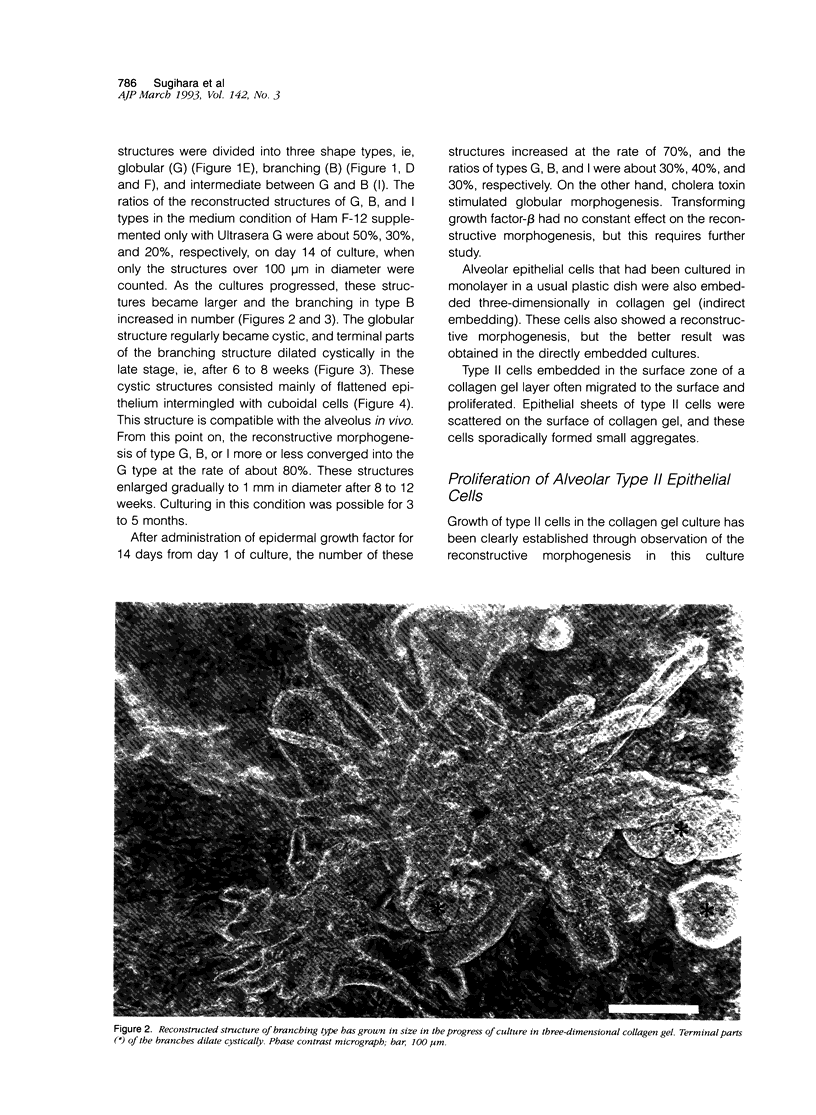
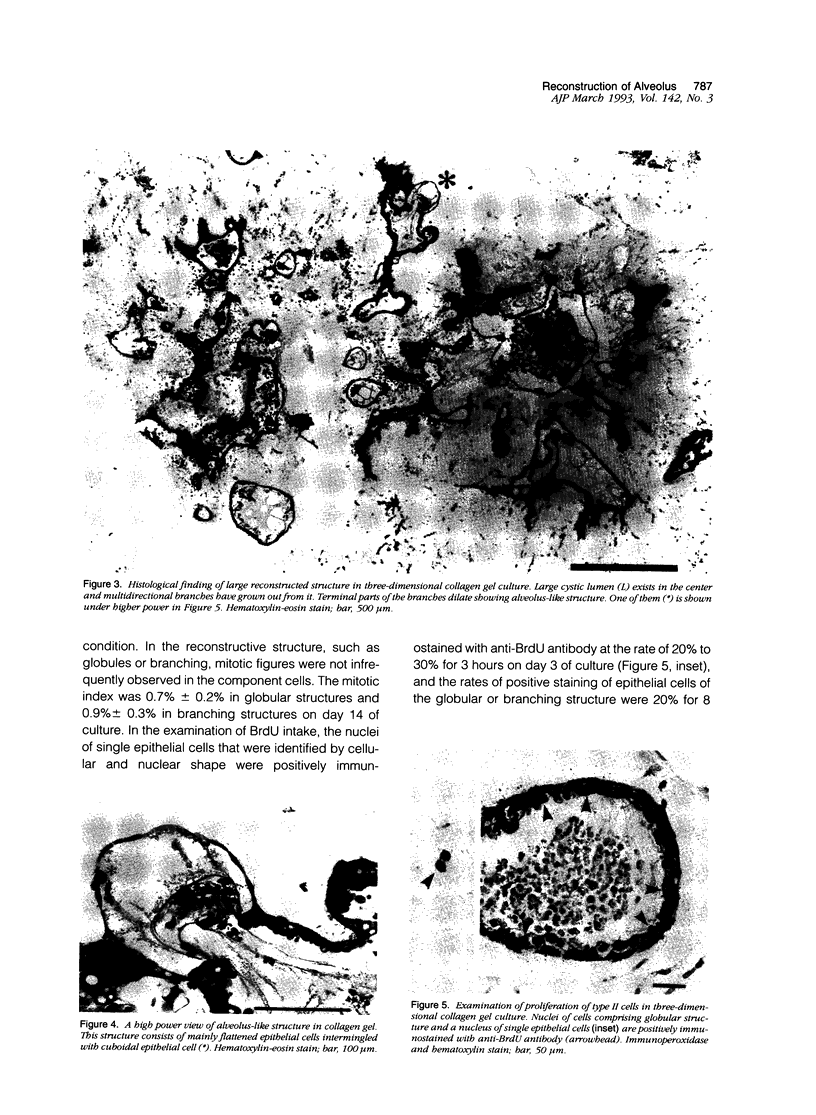
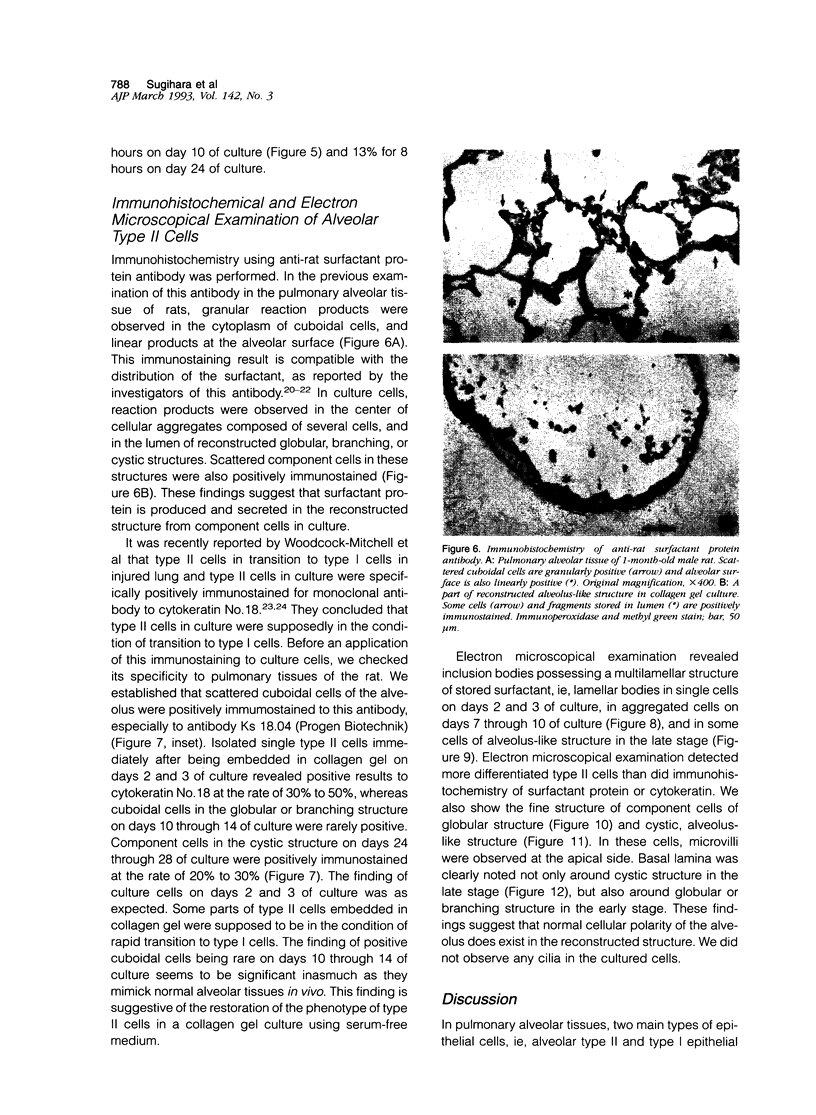

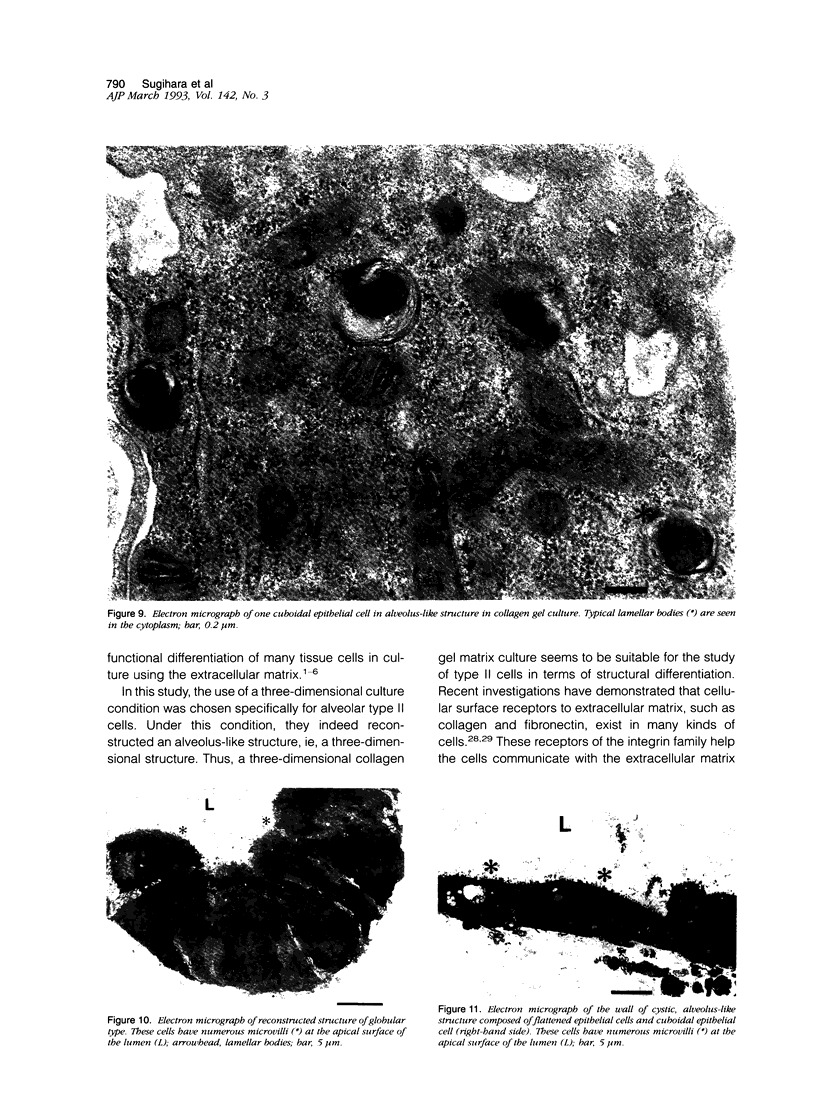


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau H., Guzowski D. E., Siddiqi Z. A., Scarpelli E. M., Bienkowski R. S. Fetal type 2 pneumocytes form alveolar-like structures and maintain long-term differentiation on extracellular matrix. J Cell Physiol. 1988 Aug;136(2):203–214. doi: 10.1002/jcp.1041360202. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Gonzalez R., Williams M. C. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986 Jul;134(1):141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G. Isolation and culture of alveolar type II cells. Am J Physiol. 1990 Apr;258(4 Pt 1):L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Williams M. C., Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta. 1988 Jun 30;970(2):146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- Durban E. M. Mouse submandibular salivary epithelial cell growth and differentiation in long-term culture: influence of the extracellular matrix. In Vitro Cell Dev Biol. 1990 Jan;26(1):33–43. doi: 10.1007/BF02624152. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githens S., Schexnayder J. A., Desai K., Patke C. L. Rat pancreatic interlobular duct epithelium: isolation and culture in collagen gel. In Vitro Cell Dev Biol. 1989 Aug;25(8):679–688. doi: 10.1007/BF02623720. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G., Leif R. C., Ingram D. J., Castro A. The use of antibody specific for bromodeoxyuridine for the immunofluorescent determination of DNA replication in single cells and chromosomes. Exp Cell Res. 1975 Oct 1;95(1):88–94. doi: 10.1016/0014-4827(75)90612-6. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Yoneda K. The type II epithelial cell of the lung. I. Method of isolation. Lab Invest. 1974 Jan;30(1):76–84. [PubMed] [Google Scholar]
- Kuroki Y., Dempo K., Akino T. Immunohistochemical study of human pulmonary surfactant apoproteins with monoclonal antibodies. Pathologic application for hyaline membrane disease. Am J Pathol. 1986 Jul;124(1):25–33. [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y., Mason R. J., Voelker D. R. Pulmonary surfactant apoprotein A structure and modulation of surfactant secretion by rat alveolar type II cells. J Biol Chem. 1988 Mar 5;263(7):3388–3394. [PubMed] [Google Scholar]
- Paine R., 3rd, Joyce-Brady M., Clement A., Brody J. S. Serum accelerates the loss of type II cell differentiation in vitro. Am J Respir Cell Mol Biol. 1990 Oct;3(4):311–323. doi: 10.1165/ajrcmb/3.4.311. [DOI] [PubMed] [Google Scholar]
- Shannon J. M., Mason R. J., Jennings S. D. Functional differentiation of alveolar type II epithelial cells in vitro: effects of cell shape, cell-matrix interactions and cell-cell interactions. Biochim Biophys Acta. 1987 Nov 12;931(2):143–156. doi: 10.1016/0167-4889(87)90200-x. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Miyamura K., Kuroki Y. Appearance of surfactant proteins, SP-A and SP-B, in developing rat lung and the effects of in vivo dexamethasone treatment. Biochim Biophys Acta. 1991 Jan 4;1081(1):53–60. doi: 10.1016/0005-2760(91)90249-h. [DOI] [PubMed] [Google Scholar]
- Sugahara K., Voelker D. R., Mason R. J. Insulin stimulates amino acid transport by alveolar type II epithelial cells in primary culture. Am Rev Respir Dis. 1987 Mar;135(3):617–621. doi: 10.1164/arrd.1987.135.3.617. [DOI] [PubMed] [Google Scholar]
- Sugihara H., Toda S., Miyabara S., Kusaba Y., Minami Y. Reconstruction of the skin in three-dimensional collagen gel matrix culture. In Vitro Cell Dev Biol. 1991 Feb;27A(2):142–146. doi: 10.1007/BF02631000. [DOI] [PubMed] [Google Scholar]
- Sugihara H., Yonemitsu N., Toda S., Miyabara S., Funatsumaru S., Matsumoto T. Unilocular fat cells in three-dimensional collagen gel matrix culture. J Lipid Res. 1988 May;29(5):691–697. [PubMed] [Google Scholar]
- Toda S., Sugihara H. Reconstruction of thyroid follicles from isolated porcine follicle cells in three-dimensional collagen gel culture. Endocrinology. 1990 Apr;126(4):2027–2034. doi: 10.1210/endo-126-4-2027. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J. L., Burkhardt A. L., Mitchell J. J., Rannels S. R., Rannels D. E., Chiu J. F., Low R. B. Keratin species in type II pneumocytes in culture and during lung injury. Am Rev Respir Dis. 1986 Sep;134(3):566–571. doi: 10.1164/arrd.1986.134.3.566. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Rannels S. R., Mitchell J., Rannels D. E., Low R. B. Modulation of keratin expression in type II pneumocytes by the extracellular matrix. Am Rev Respir Dis. 1989 Feb;139(2):343–351. doi: 10.1164/ajrccm/139.2.343. [DOI] [PubMed] [Google Scholar]
- Yang J., Richards J., Bowman P., Guzman R., Enami J., McCormick K., Hamamoto S., Pitelka D., Nandi S. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3401–3405. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Hilborn V., Hassett C., Melfi P., Byers M. J., Freeman A. E. Characterization of mouse fetal lung cells cultured on a pigskin substrate. In Vitro. 1980 May;16(5):433–445. doi: 10.1007/BF02618367. [DOI] [PubMed] [Google Scholar]