Abstract
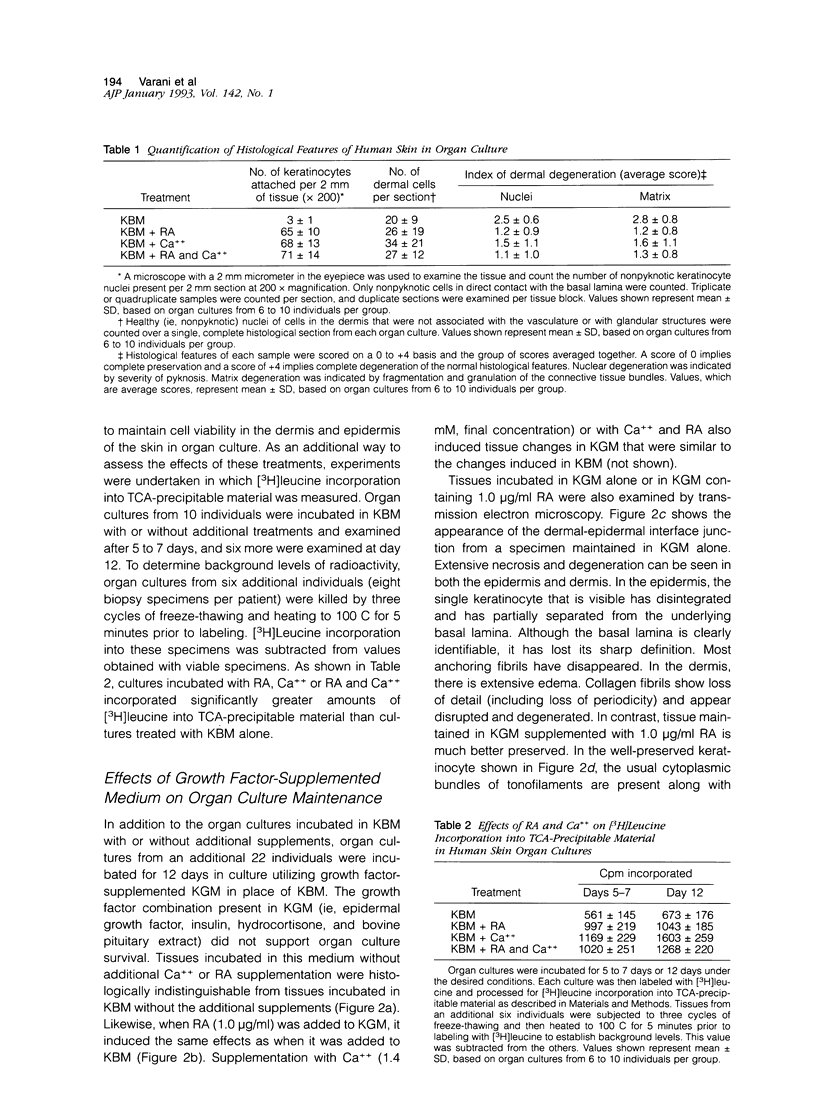
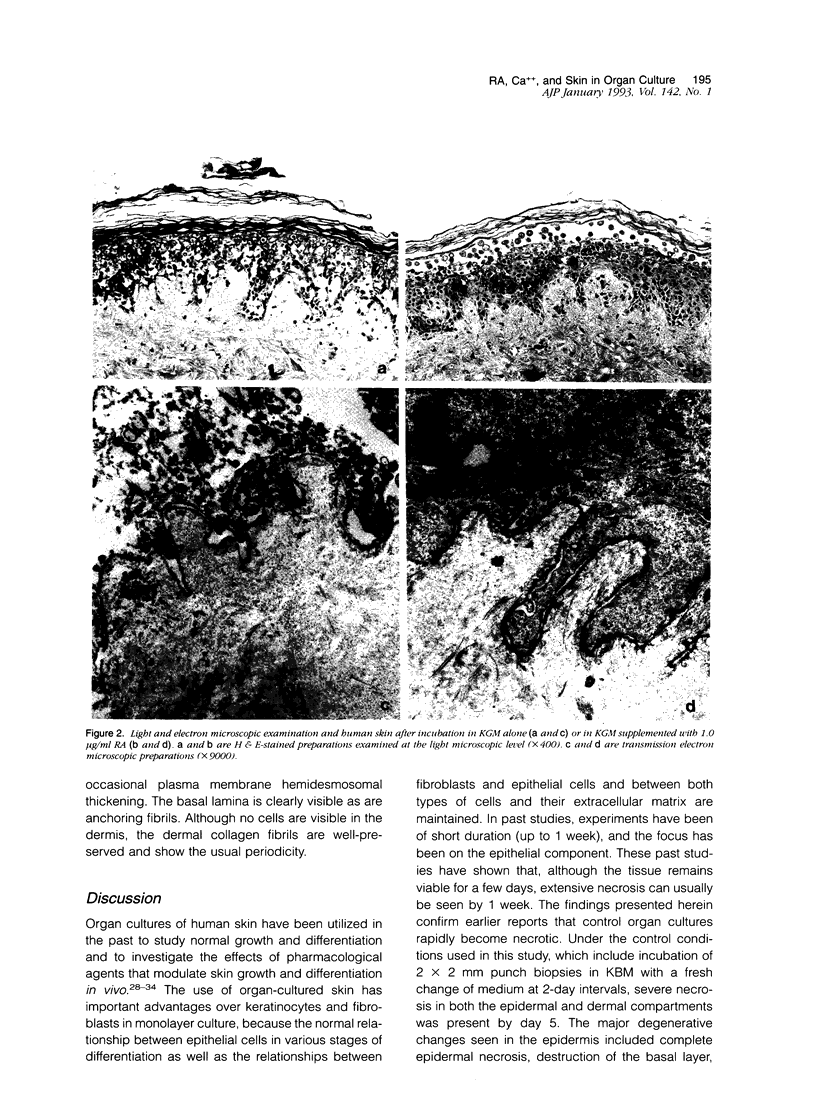
In this study, we have established an organ culture model of human skin and examined the effects of both all-trans retinoic acid (RA) and extracellular Ca++ on the epidermal and dermal components of the organ-cultured skin. Our data show that while organ cultures maintained in serum-free, growth factor-free culture medium containing 0.15 mM Ca++ degenerated rapidly, those treated with concentrations of RA that have been shown previously to stimulate fibroblast and keratinocyte proliferation in monolayer culture (J Invest Dermatol 1989, 93:449; 1990, 94:717; Am J Pathol 1990, 136:1275) demonstrated a healthy appearance for up to 12 days. Degeneration of the control cultures was characterized by separation of the epidermis from the underlying dermis, progressive cell necrosis leading to a complete absence of viable cells from both the dermal and epidermal compartments, disintegration and fibrillation of the dermal connective tissue, and a cessation of protein synthesis. RA-treated organ cultures contained large numbers of healthy-appearing cells in both the epidermal and dermal compartments. One or several layers of viable basal cells in the epidermis could be seen at least through day 12. However, the upper layers of the epidermis frequently separated from the cells in the basal layer. The dermal connective tissue was histologically well-preserved. Furthermore, the level of protein synthesis was higher in the RA-treated cultures than in the control cultures. In addition to treating organ cultures with RA, other cultures were exposed to serum-free, growth factor-free culture medium containing 1.4 mM Ca++. The presence of the elevated Ca++ concentration also preserved cellular and connective tissue structures in the dermal and epidermal compartments. In comparison to RA there was better preservation of the overall epidermal structure. The upper layers of epidermal cells did not separate from the basal cells, and the various stages of epithelial differentiation could be seen. Histologically, the dermis was well-preserved in the presence of elevated extracellular Ca++. Specimens treated with a combination of Ca++ and RA demonstrated features consistent with the features induced by each treatment separately. This included an expanded basal layer of epithelial cells and a prominent keratotic layer with a fairly orderly pattern of differentiation. The tendency of the upper epidermis to separate from the basal cells was partially mitigated. Taken together, these data indicate that both RA and extracellular Ca++ act to prevent the degeneration of human skin in organ culture but probably do so through different mechanisms.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamo S., Sasak W., Dion L. D., De Luca L. M. Studies on the mechanism of retinoid-induced adhesion of spontaneously transformed mouse fibroblasts. Acta Vitaminol Enzymol. 1983;5(1):3–10. [PubMed] [Google Scholar]
- Bhawan J., Gonzalez-Serva A., Nehal K., Labadie R., Lufrano L., Thorne E. G., Gilchrest B. A. Effects of tretinoin on photodamaged skin. A histologic study. Arch Dermatol. 1991 May;127(5):666–672. [PubMed] [Google Scholar]
- Bracke M. E., Van Larebeke N. A., Vyncke B. M., Mareel M. M. Retinoic acid modulates both invasion and plasma membrane ruffling of MCF-7 human mammary carcinoma cells in vitro. Br J Cancer. 1991 Jun;63(6):867–872. doi: 10.1038/bjc.1991.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce G. F., Bogdan N. J., Brown C. C. Retinoic acids promote the repair of the dermal damage and the effacement of wrinkles in the UVB-irradiated hairless mouse. J Invest Dermatol. 1988 Aug;91(2):175–180. doi: 10.1111/1523-1747.ep12464445. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Resing K. A., Lonsdale-Eccles J. D. Filaggrin: a keratin filament associated protein. Ann N Y Acad Sci. 1985;455:330–342. doi: 10.1111/j.1749-6632.1985.tb50420.x. [DOI] [PubMed] [Google Scholar]
- Demetriou A. A., Levenson S. M., Rettura G., Seifter E. Vitamin A and retinoic acid: induced fibroblast differentiation in vitro. Surgery. 1985 Nov;98(5):931–934. [PubMed] [Google Scholar]
- Eckert R. L., Rorke E. A. Molecular biology of keratinocyte differentiation. Environ Health Perspect. 1989 Mar;80:109–116. doi: 10.1289/ehp.8980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P. M., Fritsch P. O., Lampe M., Williams M. L., Brown B. E., Nemanic M., Grayson S. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981 Jun;44(6):531–540. [PubMed] [Google Scholar]
- Floyd E. E., Jetten A. M. Regulation of type I (epidermal) transglutaminase mRNA levels during squamous differentiation: down regulation by retinoids. Mol Cell Biol. 1989 Nov;9(11):4846–4851. doi: 10.1128/mcb.9.11.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Fuchs E., Watt F. Differentiated structural components of the keratinocyte. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):293–301. doi: 10.1101/sqb.1982.046.01.031. [DOI] [PubMed] [Google Scholar]
- Harper R. A., Burgoon T. Differential effects of retinoic acid on the growth of normal fibroblast-like cells in vitro from human, swine and rabbit skin. Cell Biol Int Rep. 1982 Feb;6(2):163–170. doi: 10.1016/0309-1651(82)90093-5. [DOI] [PubMed] [Google Scholar]
- Hein R., Mensing H., Müller P. K., Braun-Falco O., Krieg T. Effect of vitamin A and its derivatives on collagen production and chemotactic response of fibroblasts. Br J Dermatol. 1984 Jul;111(1):37–44. doi: 10.1111/j.1365-2133.1984.tb04014.x. [DOI] [PubMed] [Google Scholar]
- Iwata M., Iwata S., Everett M. A., Fuller B. B. Hormonal stimulation of tyrosinase activity in human foreskin organ cultures. In Vitro Cell Dev Biol. 1990 Jun;26(6):554–560. doi: 10.1007/BF02624203. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., George M. A., Pettit G. R., Herald C. L., Rearick J. I. Action of phorbol esters, bryostatins, and retinoic acid on cholesterol sulfate synthesis: relation to the multistep process of differentiation in human epidermal keratinocytes. J Invest Dermatol. 1989 Jul;93(1):108–115. doi: 10.1111/1523-1747.ep12277374. [DOI] [PubMed] [Google Scholar]
- Jetten A. M. Multi-stage program of differentiation in human epidermal keratinocytes: regulation by retinoids. J Invest Dermatol. 1990 Nov;95(5 Suppl):44S–46S. doi: 10.1111/1523-1747.ep12505757. [DOI] [PubMed] [Google Scholar]
- Kato S., De Luca L. M. Retinoic acid modulates attachment of mouse fibroblasts to laminin substrates. Exp Cell Res. 1987 Dec;173(2):450–462. doi: 10.1016/0014-4827(87)90285-0. [DOI] [PubMed] [Google Scholar]
- Kligman A. M., Grove G. L., Hirose R., Leyden J. J. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Duo C. H., Kligman A. M. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12(2):139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- Kligman L. H. Effects of all-trans-retinoic acid on the dermis of hairless mice. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):779-85, 884-7. doi: 10.1016/s0190-9622(86)70234-x. [DOI] [PubMed] [Google Scholar]
- Kondo S., Hozumi Y., Aso K. Organ culture of psoriatic lesions: appearance of granular layers in vitamin A-free culture media. J Invest Dermatol. 1992 May;98(5):753–757. doi: 10.1111/1523-1747.ep12499945. [DOI] [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix A., Anderson G. D., Lippman M. E. Retinoids and cultured human fibroblasts. Effects on cell growth and presence of cellular retinoic acid-binding protein. Exp Cell Res. 1980 Dec;130(2):339–344. doi: 10.1016/0014-4827(80)90010-5. [DOI] [PubMed] [Google Scholar]
- Merrick D. T., Blanton R. A., Gown A. M., McDougall J. K. Altered expression of proliferation and differentiation markers in human papillomavirus 16 and 18 immortalized epithelial cells grown in organotypic culture. Am J Pathol. 1992 Jan;140(1):167–177. [PMC free article] [PubMed] [Google Scholar]
- Milstone L. M., McGuire J., LaVigne J. F. Retinoic acid causes premature desquamation of cells from confluent cultures of stratified squamous epithelia. J Invest Dermatol. 1982 Oct;79(4):253–260. doi: 10.1111/1523-1747.ep12500073. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Tada H., Kobayashi N., Yamji M., Shirai T., Ohnishi T. Induction of the 72-kD heat shock protein in organ-cultured normal human skin. J Invest Dermatol. 1992 May;98(5):786–790. doi: 10.1111/1523-1747.ep12499953. [DOI] [PubMed] [Google Scholar]
- Orfanos C. E., Runne U. Tissue changes in psoriatic plaques after oral administration of retinoid. Dermatologica. 1978;157 (Suppl 1):19–25. doi: 10.1159/000250880. [DOI] [PubMed] [Google Scholar]
- Priestley G. C. Proliferation and glycosaminoglycans secretion in fibroblasts from psoriatic skin: differential responses to retinoids. Br J Dermatol. 1987 Nov;117(5):575–583. doi: 10.1111/j.1365-2133.1987.tb07489.x. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977 Jun;11(2):417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal D. S., Roop D. R., Huff C. A., Weiss J. S., Ellis C. N., Hamilton T., Voorhees J. J., Yuspa S. H. Changes in photo-aged human skin following topical application of all-trans retinoic acid. J Invest Dermatol. 1990 Nov;95(5):510–515. doi: 10.1111/1523-1747.ep12504718. [DOI] [PubMed] [Google Scholar]
- Schuger L., O'Shea K. S., Nelson B. B., Varani J. Organotypic arrangement of mouse embryonic lung cells on a basement membrane extract: involvement of laminin. Development. 1990 Dec;110(4):1091–1099. doi: 10.1242/dev.110.4.1091. [DOI] [PubMed] [Google Scholar]
- Schuger L., O'Shea S., Rheinheimer J., Varani J. Laminin in lung development: effects of anti-laminin antibody in murine lung morphogenesis. Dev Biol. 1990 Jan;137(1):26–32. doi: 10.1016/0012-1606(90)90004-3. [DOI] [PubMed] [Google Scholar]
- Schuger L., Skubitz A. P., O'Shea K. S., Chang J. F., Varani J. Identification of laminin domains involved in branching morphogenesis: effects of anti-laminin monoclonal antibodies on mouse embryonic lung development. Dev Biol. 1991 Aug;146(2):531–541. doi: 10.1016/0012-1606(91)90254-z. [DOI] [PubMed] [Google Scholar]
- Tammi R. A histometric and autoradiographic study of hydrocortisone action in cultured human epidermis. Br J Dermatol. 1981 Oct;105(4):383–389. doi: 10.1111/j.1365-2133.1981.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Tammi R., Jansén C. T., Tammi M. Effects of retinoic acid on adult human epidermis in whole skin organ culture. Arch Dermatol Res. 1985;277(4):276–283. doi: 10.1007/BF00509080. [DOI] [PubMed] [Google Scholar]
- Tammi R., Jansén C. Effect of serum and oxygen tension on human skin organ culture: a histometric analysis. Acta Derm Venereol. 1980;60(3):223–228. [PubMed] [Google Scholar]
- Tammi R., Santti R. Ultrastructural study of hydrocortisone action in cultured human epidermis. Br J Dermatol. 1982 Jan;106(1):65–75. doi: 10.1111/j.1365-2133.1982.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Thacher S. M., Rice R. H. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985 Mar;40(3):685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- Varani J., Gibbs D. F., Inman D. R., Shah B., Fligiel S. E., Voorhees J. J. Inhibition of epithelial cell adhesion by retinoic acid. Relationship to reduced extracellular matrix production and alterations in Ca2+ levels. Am J Pathol. 1991 Apr;138(4):887–895. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Mitra R. S., Gibbs D., Phan S. H., Dixit V. M., Mitra R., Jr, Wang T., Siebert K. J., Nickoloff B. J., Voorhees J. J. All-trans retinoic acid stimulates growth and extracellular matrix production in growth-inhibited cultured human skin fibroblasts. J Invest Dermatol. 1990 May;94(5):717–723. doi: 10.1111/1523-1747.ep12876294. [DOI] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Dixit V. M., Mitra R. S., Voorhees J. J. All-trans retinoic acid stimulates growth of adult human keratinocytes cultured in growth factor-deficient medium, inhibits production of thrombospondin and fibronectin, and reduces adhesion. J Invest Dermatol. 1989 Oct;93(4):449–454. doi: 10.1111/1523-1747.ep12284020. [DOI] [PubMed] [Google Scholar]
- Varani J., Shayevitz J., Perry D., Mitra R. S., Nickoloff B. J., Voorhees J. J. Retinoic acid stimulation of human dermal fibroblast proliferation is dependent on suboptimal extracellular Ca2+ concentration. Am J Pathol. 1990 Jun;136(6):1275–1281. [PMC free article] [PubMed] [Google Scholar]
- Weinstein G. D., Nigra T. P., Pochi P. E., Savin R. C., Allan A., Benik K., Jeffes E., Lufrano L., Thorne E. G. Topical tretinoin for treatment of photodamaged skin. A multicenter study. Arch Dermatol. 1991 May;127(5):659–665. [PubMed] [Google Scholar]
- Weiss J. S., Ellis C. N., Headington J. T., Tincoff T., Hamilton T. A., Voorhees J. J. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988 Jan 22;259(4):527–532. [PubMed] [Google Scholar]
- Williams M. L., Elias P. M. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981 Oct;117(10):611–619. [PubMed] [Google Scholar]