Abstract
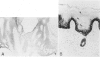
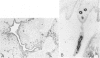
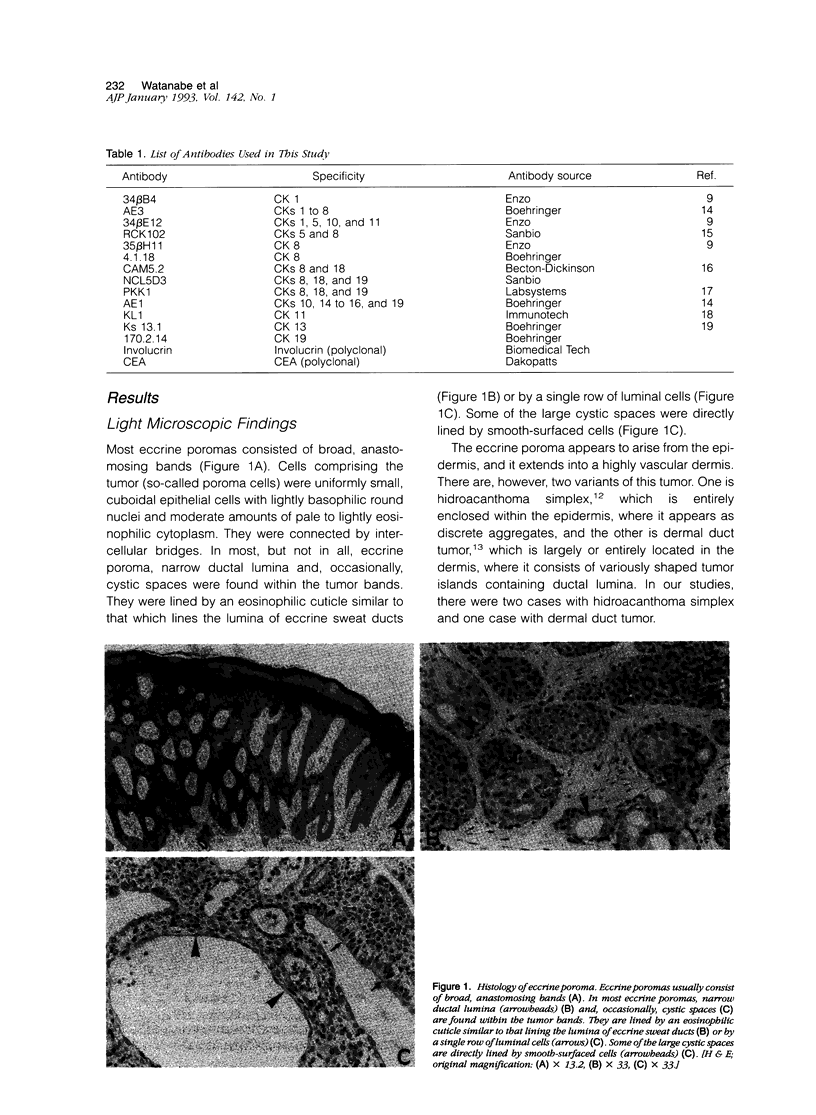
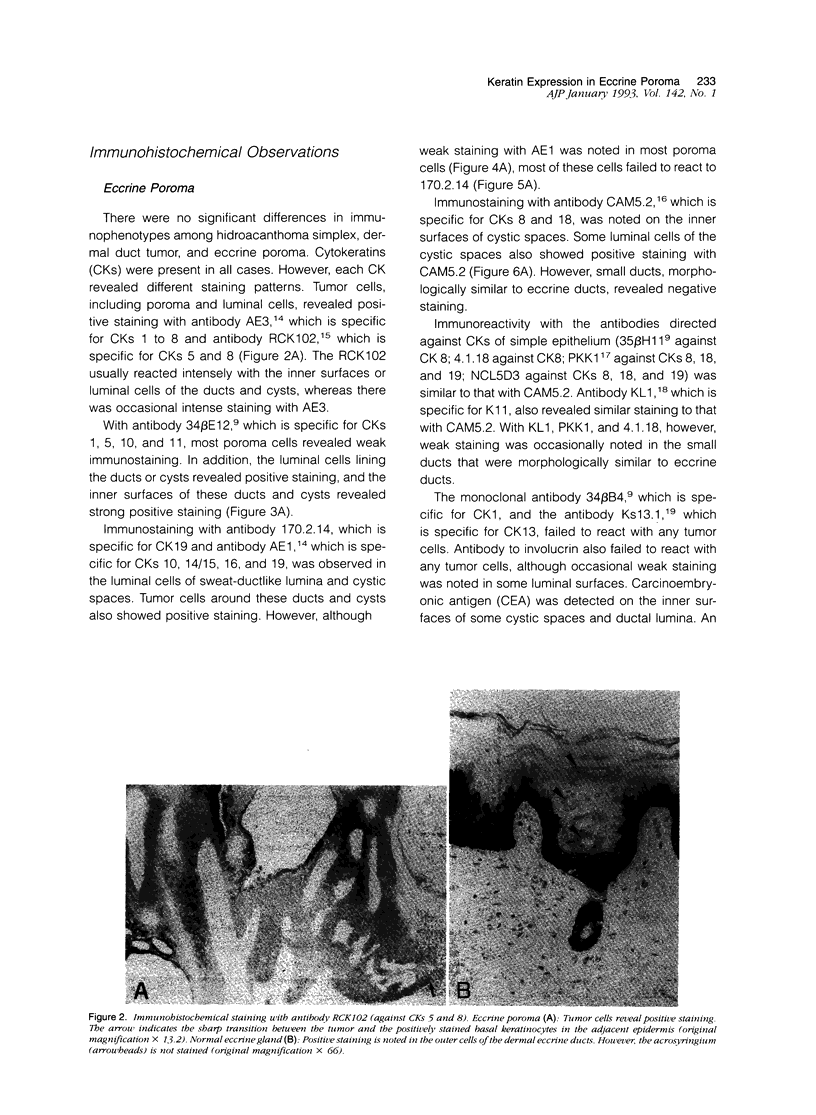
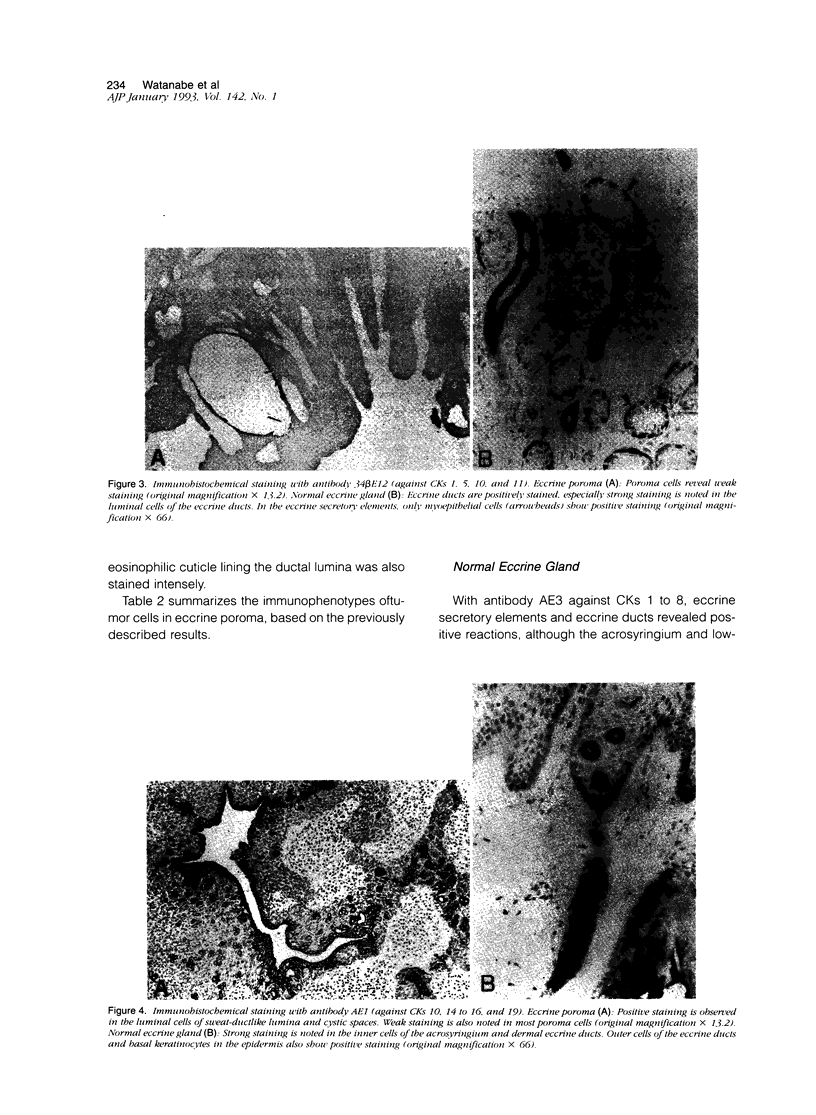
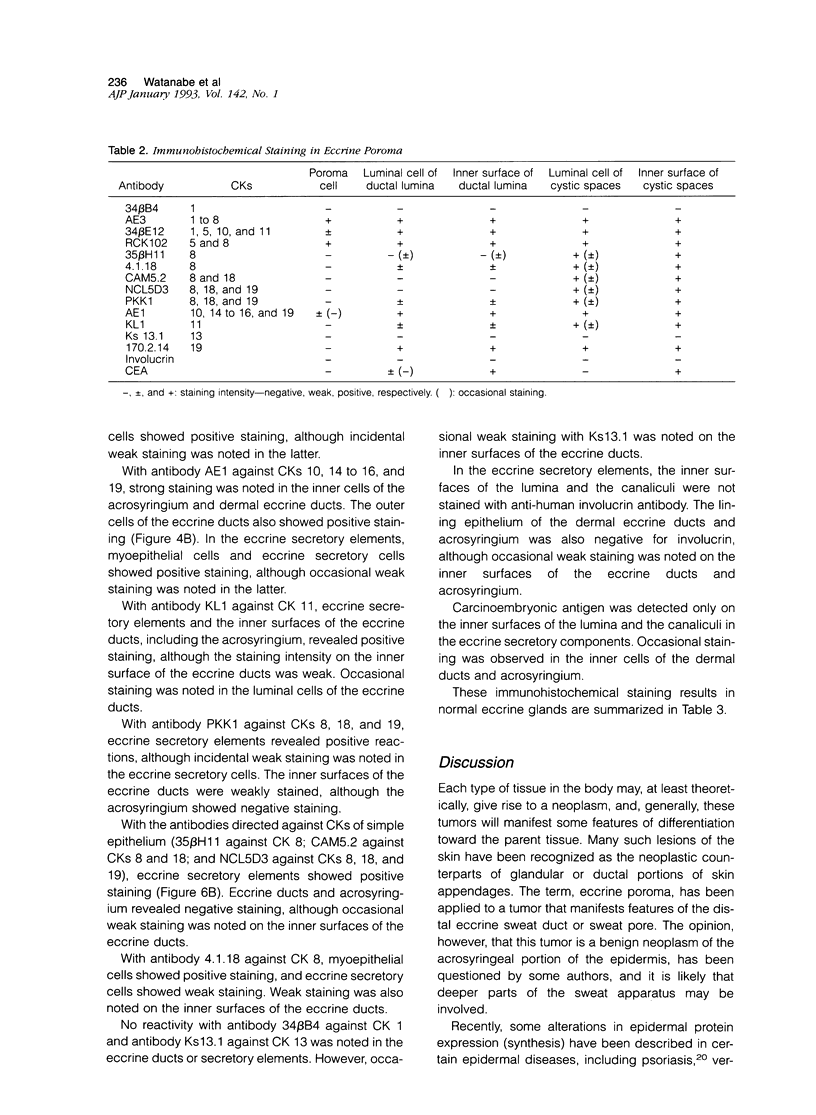
Although eccrine poroma has been thought of as a neoplasm of the intradermal eccrine duct, this interpretation has not been entirely confirmed. In this study, twenty-five cases of eccrine poroma were retrieved and analyzed by immunohistochemical techniques, using various kinds of monoclonal antikeratin antibodies. Comparative immunohistochemical observations of eccrine poroma and normal eccrine glands revealed that the poroma cells expressed immunophenotypes similar to those of the basal cells of dermal eccrine ducts. Sweat-ductlike structures showed similar staining patterns to those observed in the inner cells of dermal eccrine ducts. Some cystic spaces were similar to those observed in the secretory cells of eccrine glands. Eccrine poroma is, therefore, speculated to originate via the proliferation and expansion of the basal cells of eccrine ducts, although it is very difficult to prove the histogenesis. Some tumor cells may differentiate toward inner cells of the eccrine ducts, forming ductal lumina, whereas other tumor cells differentiate toward eccrine secretory regions, forming some cystic spaces.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battifora H. Recent progress in the immunohistochemistry of solid tumors. Semin Diagn Pathol. 1984 Nov;1(4):251–271. [PubMed] [Google Scholar]
- Broers J. L., Carney D. N., Klein Rot M., Schaart G., Lane E. B., Vooijs G. P., Ramaekers F. C. Intermediate filament proteins in classic and variant types of small cell lung carcinoma cell lines: a biochemical and immunochemical analysis using a panel of monoclonal and polyclonal antibodies. J Cell Sci. 1986 Jul;83:37–60. doi: 10.1242/jcs.83.1.37. [DOI] [PubMed] [Google Scholar]
- COBURN J. G., SMITH J. L. Hidroacanthoma simplex; an assessment of a selected group of intraepidermal basal cell epitheliomata and of their malignant homologues. Br J Dermatol. 1956 Dec;68(12):400–418. doi: 10.1111/j.1365-2133.1956.tb12776.x. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Debus E., Moll R., Franke W. W., Weber K., Osborn M. Immunohistochemical distinction of human carcinomas by cytokeratin typing with monoclonal antibodies. Am J Pathol. 1984 Jan;114(1):121–130. [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN P., PINKUS H., ROGIN J. R. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956 Nov;74(5):511–521. [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO K., LEVER W. F. ECCRINE POROMA; HISTOCHEMICAL AND ELECTRON MICROSCOPIC STUDIES. J Invest Dermatol. 1964 Oct;43:237–247. [PubMed] [Google Scholar]
- Hashimoto K., Lever W. F. Histogenesis of skin appendage tumors. Arch Dermatol. 1969 Sep;100(3):356–369. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hu C. H., Marques A. S., Winkelmann R. K. Dermal duct tumor: a histochemical and electron microscopic study. Arch Dermatol. 1978 Nov;114(11):1659–1664. doi: 10.1001/archderm.114.11.1659. [DOI] [PubMed] [Google Scholar]
- Makin C. A., Bobrow L. G., Bodmer W. F. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984 Sep;37(9):975–983. doi: 10.1136/jcp.37.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Virtanen I., Talerman A. Intermediate filament proteins in human testis and testicular germ-cell tumors. Am J Pathol. 1985 Sep;120(3):402–410. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Achtstätter T., Becht E., Balcarova-Ständer J., Ittensohn M., Franke W. W. Cytokeratins in normal and malignant transitional epithelium. Maintenance of expression of urothelial differentiation features in transitional cell carcinomas and bladder carcinoma cell culture lines. Am J Pathol. 1988 Jul;132(1):123–144. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Volc-Platzer B., Krepler R. Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol. 1982 Oct;95(1):285–295. doi: 10.1083/jcb.95.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDERSON K. V., RYAN E. A. The histochemistry of eccrine poroma. Br J Dermatol. 1963 Feb;75:86–88. doi: 10.1111/j.1365-2133.1963.tb13943.x. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Goodman M. L., Bhan A. K., Pilch B. Z., Chen L. B., Sun T. T. Immunohistochemical study of nasopharyngeal carcinoma using monoclonal keratin antibodies. Am J Pathol. 1984 Oct;117(1):53–63. [PMC free article] [PubMed] [Google Scholar]
- Staquet M. J., Viac J., Thivolet J. Keratin polypeptide modifications induced by human papilloma viruses (HPV). Arch Dermatol Res. 1981;271(1):83–90. doi: 10.1007/BF00417391. [DOI] [PubMed] [Google Scholar]
- Viac J., Reano A., Brochier J., Staquet M. J., Thivolet J. Reactivity pattern of a monoclonal antikeratin antibody (KL1). J Invest Dermatol. 1983 Oct;81(4):351–354. doi: 10.1111/1523-1747.ep12519941. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Wagatsuma K., Ichikawa E., Takahashi H. Abnormal distribution of epidermal protein antigens in psoriatic epidermis. J Dermatol. 1991 Mar;18(3):143–151. doi: 10.1111/j.1346-8138.1991.tb03057.x. [DOI] [PubMed] [Google Scholar]
- Winkelmann R. K., McLeod W. A. The dermal duct tumor. Arch Dermatol. 1966 Jul;94(1):50–55. [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., Ponec M., Huiskens-van der, Mey C., Warnaar S. O. Monoclonal antibodies with different specificities against cytokeratins. An immunohistochemical study of normal tissues and tumors. Am J Pathol. 1984 Jan;114(1):9–17. [PMC free article] [PubMed] [Google Scholar]