Abstract
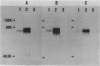
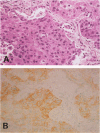
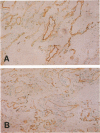
Thrombomodulin (TM), which usually exists in vascular endothelial cells and exerts an anticoagulant activity, was detected by Western blot analyses and immunocytochemical staining using three anti-TM monoclonal antibodies in cultured cell lines derived from a squamous cell carcinoma and an adenocarcinoma of the lung, but was not detected in a cell line derived from a small cell carcinoma. Functional assays indicated that TM detected in these cells was functionally active. The presence of TM in 22 specimens of surgically removed lung cancer tissue was also examined by an immunohistochemical method. TM was present along the cell membranes in 4 (36%) of 11 squamous cell carcinomas examined, but was not detected in 10 adenocarcinomas and 1 large cell carcinoma examined. Because TM is identical to fetomodulin, which modulates embryogenesis, the authors have concluded that TM is an oncodevelopmental antigen. The authors have also suggested that functionally active TM on lung cancer cells may modulate cancer cell behaviors in such ways as exhibiting anticoagulant activity.
Full text
PDF

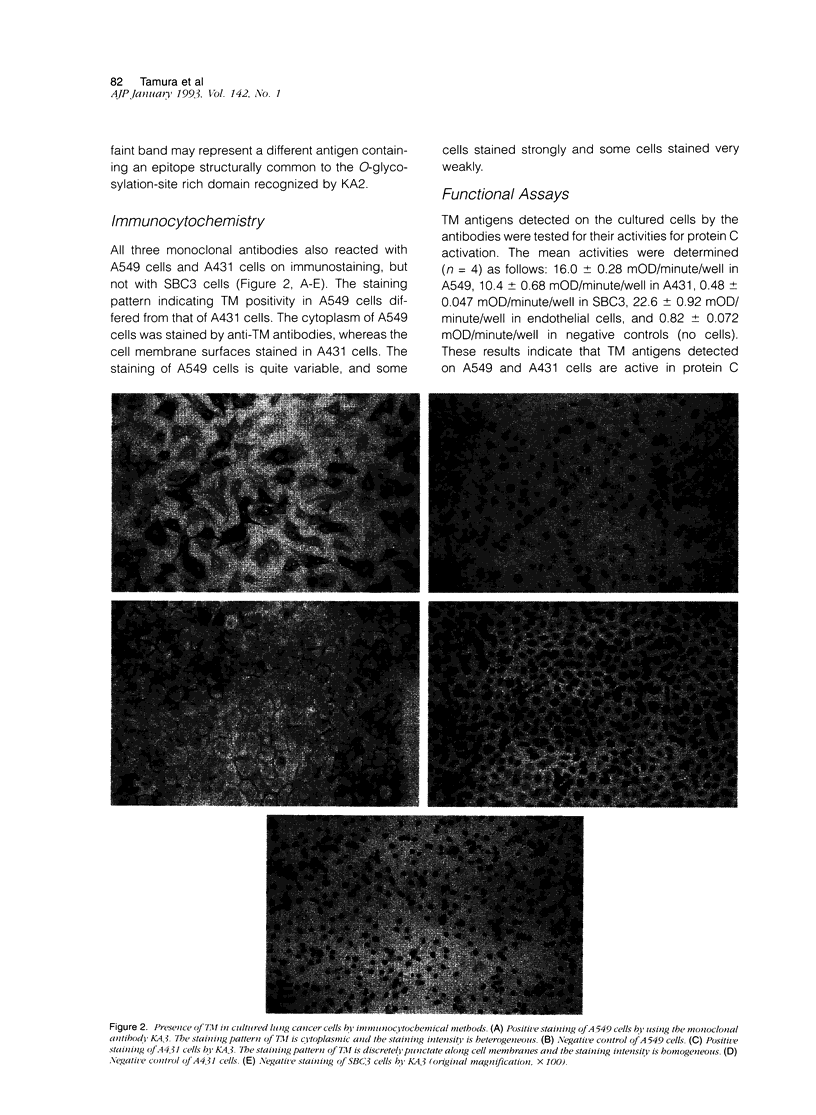
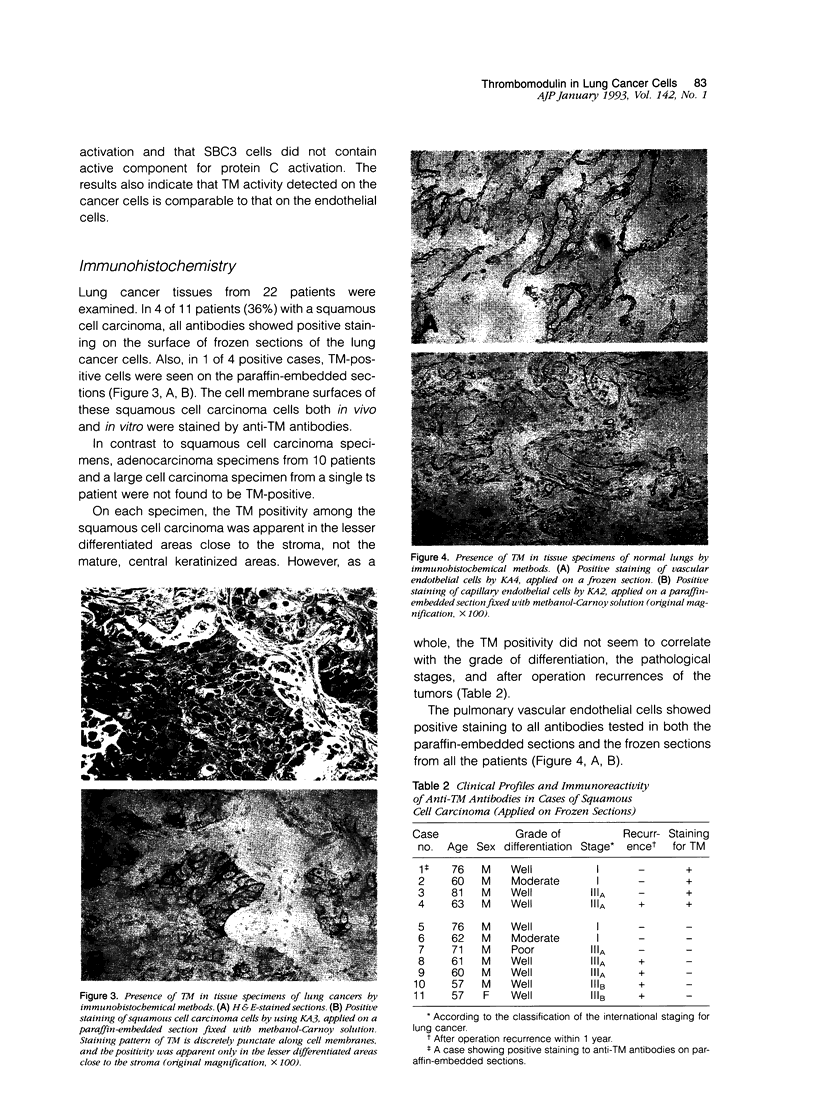


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boffa M. C., Burke B., Haudenschild C. C. Preservation of thrombomodulin antigen on vascular and extravascular surfaces. J Histochem Cytochem. 1987 Nov;35(11):1267–1276. doi: 10.1177/35.11.2821107. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Senger D. R., Dvorak A. M. Fibrin as a component of the tumor stroma: origins and biological significance. Cancer Metastasis Rev. 1983;2(1):41–73. doi: 10.1007/BF00046905. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon N. L., Carroll R. C., Esmon C. T. Thrombomodulin blocks the ability of thrombin to activate platelets. J Biol Chem. 1983 Oct 25;258(20):12238–12242. [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Fenderson B. A., Zehavi U., Hakomori S. A multivalent lacto-N-fucopentaose III-lysyllysine conjugate decompacts preimplantation mouse embryos, while the free oligosaccharide is ineffective. J Exp Med. 1984 Nov 1;160(5):1591–1596. doi: 10.1084/jem.160.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y., Steiner M., Baldini M. G. Characterization of the platelet-aggregating activity of tumor cells. Cancer Res. 1980 Apr;40(4):1217–1222. [PubMed] [Google Scholar]
- Hayashi T., Zushi M., Yamamoto S., Suzuki K. Further localization of binding sites for thrombin and protein C in human thrombomodulin. J Biol Chem. 1990 Nov 25;265(33):20156–20159. [PubMed] [Google Scholar]
- Hirokawa K., Aoki N. Up-regulation of thrombomodulin in human umbilical vein endothelial cells in vitro. J Biochem. 1990 Nov;108(5):839–845. doi: 10.1093/oxfordjournals.jbchem.a123290. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Imada M., Imada S., Iwasaki H., Kume A., Yamaguchi H., Moore E. E. Fetomodulin: marker surface protein of fetal development which is modulatable by cyclic AMP. Dev Biol. 1987 Aug;122(2):483–491. doi: 10.1016/0012-1606(87)90312-5. [DOI] [PubMed] [Google Scholar]
- Imada S., Yamaguchi H., Nagumo M., Katayanagi S., Iwasaki H., Imada M. Identification of fetomodulin, a surface marker protein of fetal development, as thrombomodulin by gene cloning and functional assays. Dev Biol. 1990 Jul;140(1):113–122. doi: 10.1016/0012-1606(90)90058-q. [DOI] [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Nudelman E., Levery S. B., Hakomori S. A series of human erythrocyte glycosphingolipids reacting to the monoclonal antibody directed to a developmentally regulated antigen SSEA-1. J Biol Chem. 1982 Dec 25;257(24):14865–14874. [PubMed] [Google Scholar]
- Kimura S., Nagoya T., Aoki N. Monoclonal antibodies to human thrombomodulin whose binding is calcium dependent. J Biochem. 1989 Mar;105(3):478–483. doi: 10.1093/oxfordjournals.jbchem.a122690. [DOI] [PubMed] [Google Scholar]
- Koyama T., Parkinson J. F., Aoki N., Bang N. U., Müller-Berghaus G., Preissner K. T. Relationship between post-translational glycosylation and anticoagulant function of secretable recombinant mutants of human thrombomodulin. Br J Haematol. 1991 Aug;78(4):515–522. doi: 10.1111/j.1365-2141.1991.tb04481.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maruyama I., Bell C. E., Majerus P. W. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985 Aug;101(2):363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M., Zenita K., Tanaka O., Okada Y., Kannagi R. Stage-specific expression of SSEA-1-related antigens in the developing lung of human embryos and its relation to the distribution of these antigens in lung cancers. Cancer Res. 1988 Dec 15;48(24 Pt 1):7150–7158. [PubMed] [Google Scholar]
- Moll R., Cowin P., Kapprell H. P., Franke W. W. Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest. 1986 Jan;54(1):4–25. [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T. Functional properties of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1981 Jun 10;256(11):5532–5535. [PubMed] [Google Scholar]
- Patthy L. Detecting distant homologies of mosaic proteins. Analysis of the sequences of thrombomodulin, thrombospondin complement components C9, C8 alpha and C8 beta, vitronectin and plasma cell membrane glycoprotein PC-1. J Mol Biol. 1988 Aug 20;202(4):689–696. doi: 10.1016/0022-2836(88)90550-5. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Ambrogio C., Gasic G., Karpatkin S. Inhibition of the platelet-aggregating activity of two human adenocarcinomas of the colon and an anaplastic murine tumor with a specific thrombin inhibitor, dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Cancer Res. 1981 Nov;41(11 Pt 1):4535–4539. [PubMed] [Google Scholar]
- Said J. W., Nash G., Tepper G., Banks-Schlegel S. Keratin proteins and carcinoembryonic antigen in lung carcinoma: an immunoperoxidase study of fifty-four cases, with ultrastructural correlations. Hum Pathol. 1983 Jan;14(1):70–76. doi: 10.1016/s0046-8177(83)80048-3. [DOI] [PubMed] [Google Scholar]
- Sala N., Owen W. G., Collen D. A functional assay of protein C in human plasma. Blood. 1984 Mar;63(3):671–675. [PubMed] [Google Scholar]
- Salem H. H., Maruyama I., Ishii H., Majerus P. W. Isolation and characterization of thrombomodulin from human placenta. J Biol Chem. 1984 Oct 10;259(19):12246–12251. [PubMed] [Google Scholar]
- Shi Z. R., Tsao D., Kim Y. S. Subcellular distribution, synthesis, and release of carcinoembryonic antigen in cultured human colon adenocarcinoma cell lines. Cancer Res. 1983 Sep;43(9):4045–4049. [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kusumoto H., Deyashiki Y., Nishioka J., Maruyama I., Zushi M., Kawahara S., Honda G., Yamamoto S., Horiguchi S. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987 Jul;6(7):1891–1897. doi: 10.1002/j.1460-2075.1987.tb02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Nishioka J., Hayashi T., Kosaka Y. Functionally active thrombomodulin is present in human platelets. J Biochem. 1988 Oct;104(4):628–632. doi: 10.1093/oxfordjournals.jbchem.a122523. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesner R. Spectrum of lung cancer and ectopic hormones. Pathol Annu. 1978;13(Pt 1):207–240. [PubMed] [Google Scholar]
- Yonezawa S., Maruyama I., Sakae K., Igata A., Majerus P. W., Sato E. Thrombomodulin as a marker for vascular tumors. Comparative study with factor VIII and Ulex europaeus I lectin. Am J Clin Pathol. 1987 Oct;88(4):405–411. doi: 10.1093/ajcp/88.4.405. [DOI] [PubMed] [Google Scholar]
- Yonezawa S., Maruyama I., Tanaka S., Nakamura T., Sato E. Immunohistochemical localization of thrombomodulin in chorionic diseases of the uterus and choriocarcinoma of the stomach. A comparative study with the distribution of human chorionic gonadotropin. Cancer. 1988 Aug 1;62(3):569–576. doi: 10.1002/1097-0142(19880801)62:3<569::aid-cncr2820620322>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]