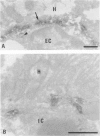
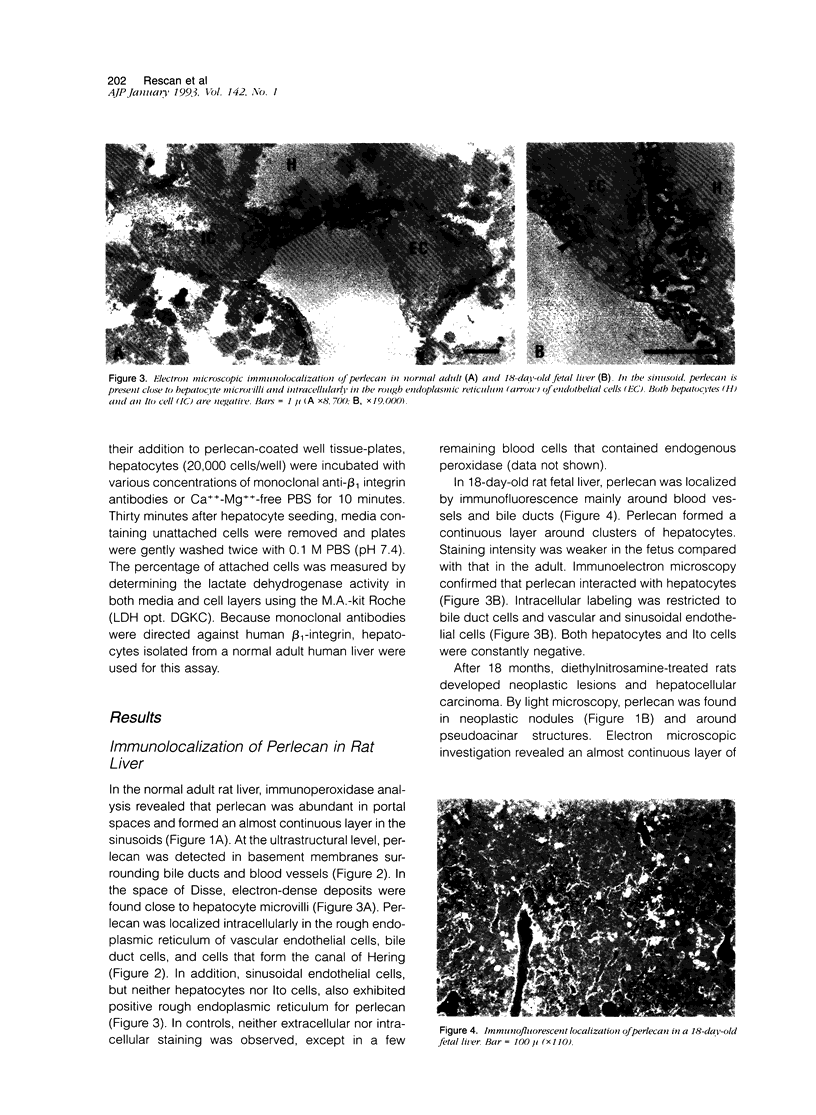
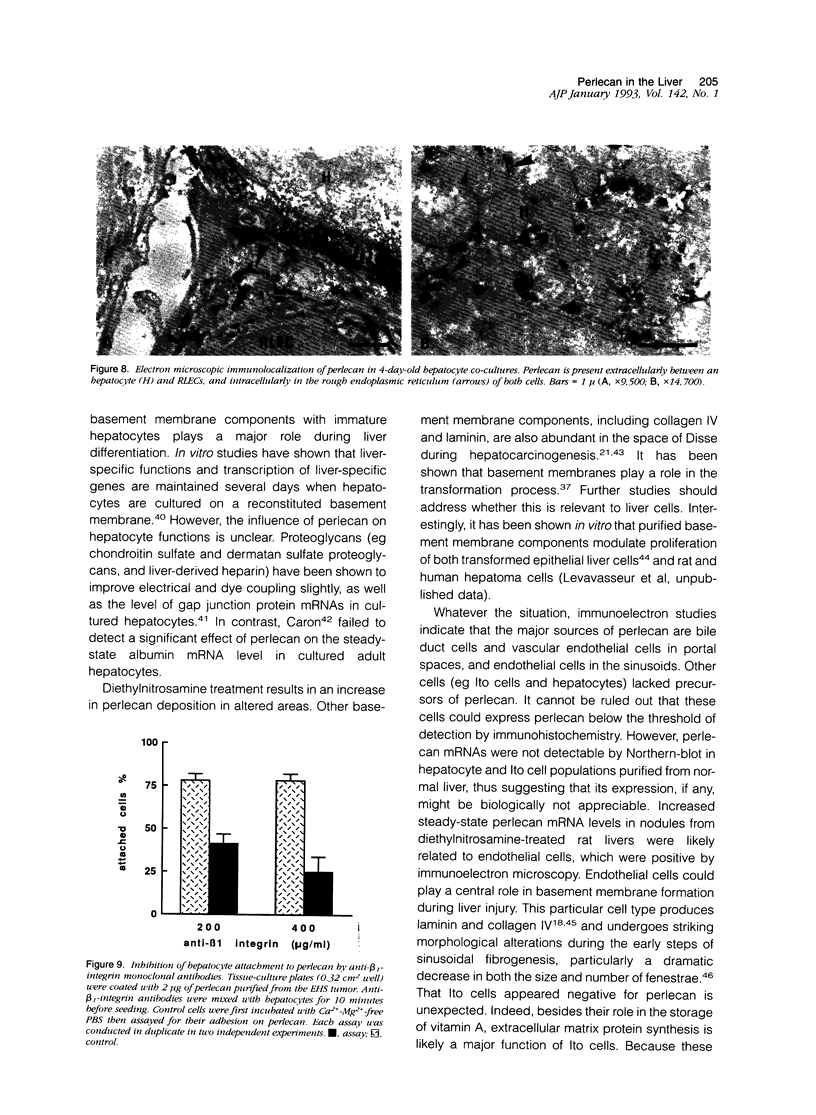
Abstract
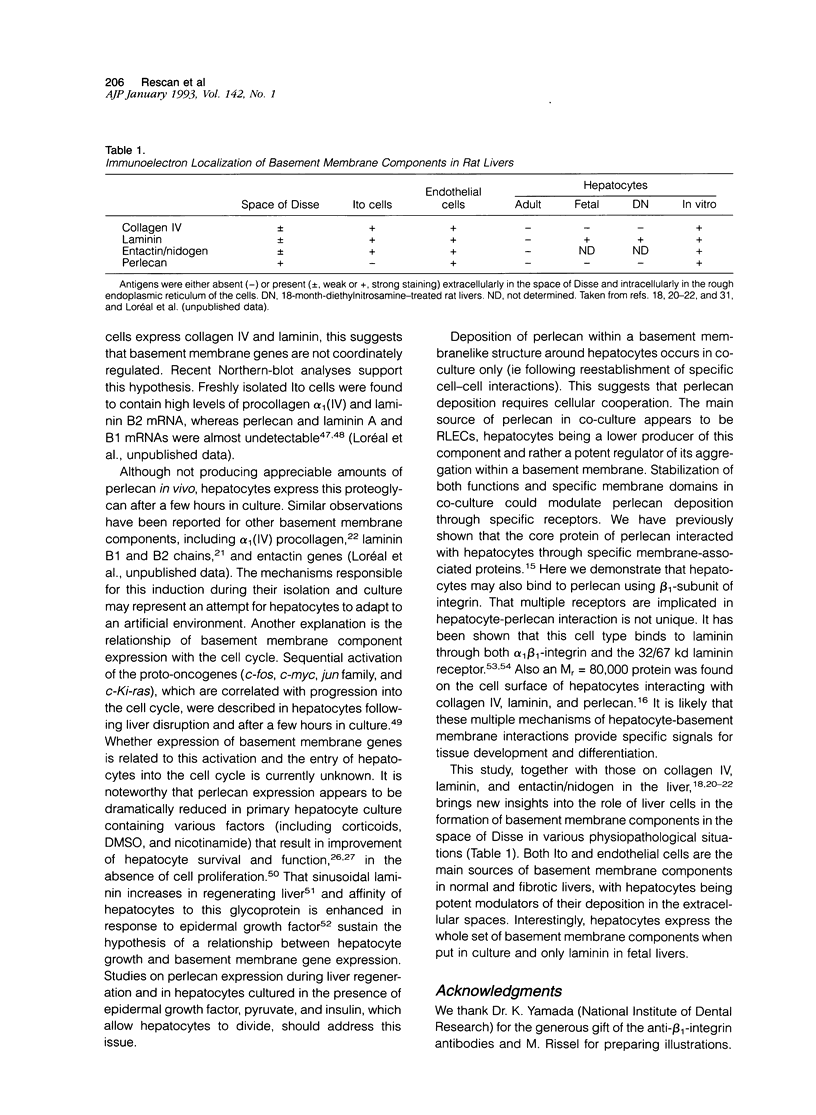
Basement membranes contain three major components (ie collagen IV, laminin, and the heparan sulfate proteoglycan termed perlecan). Although the distribution and origin of both collagen IV and laminin have been well documented in the liver, perlecan has been poorly investigated, so far. We have studied the distribution and cellular origin of perlecan in rat livers in various conditions as well as in hepatocyte primary culture. By immunolocalization in both adult and 18-day-old fetal liver, perlecan was found in portal spaces, around central veins, and throughout the lobule. Immunoelectron microscopy revealed its presence at the level of basement membranes surrounding bile ducts and blood vessels, and in the space of Disse discontinuously interacting with hepatocyte microvilli. Precursors of perlecan were detected in the rough endoplasmic reticulum of bile duct cells and both vascular and sinusoidal endothelial cells. Both hepatocytes and Ito cells were negative. Northern-blot analysis confirmed the lack of appreciable expression of perlecan in hepatocytes isolated from either fetal or adult livers. In 18-month-diethylnitrosamine-treated rat liver, perlecan was abundant in neoplastic nodules. Electron microscopic investigation revealed an almost continuous layer of perlecan in the space of Disse and intracellular staining in sinusoidal endothelial cells, only. Perlecan mRNAs were detectable in malignant nodules, and absent in hepatocytes from nontumorous areas. Hepatocytes expressed high levels of perlecan mRNAs only when put in culture. This expression was reduced in conditions that allow improvement of hepatocyte survival and function (ie addition of corticoids, dimethylsulfoxide or nicotinamide to the medium, or in coculture with liver epithelial cells from biliary origin). Immunolocalization by light and electron microscopy showed that deposition of the proteoglycan occurred in coculture, in basement membranelike structures located around hepatocyte cords. In vitro attachment assay of hepatocytes on purified perlecan substrate indicated that these cells may interact with the proteoglycan through integrins which belong to the beta 1 family. These data suggest that deposition of perlecan in the space of Disse requires cellular cooperation. This article on perlecan, the third major component of hepatic basement membranes, shows a unique cellular origin in the liver and, as found for both collagen IV and laminin, an expression in adult hepatocytes when place in culture.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Caulfield J. P. Distribution of laminin within rat and mouse renal, splenic, intestinal, and hepatic basement membranes identified after the intravenous injection of heterologous antilaminin IgG. Lab Invest. 1985 Feb;52(2):169–181. [PubMed] [Google Scholar]
- Albrechtsen R., Wewer U. M., Thorgeirsson S. S. De novo deposition of laminin-positive basement membrane in vitro by normal hepatocytes and during hepatocarcinogenesis. Hepatology. 1988 May-Jun;8(3):538–546. doi: 10.1002/hep.1840080318. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Arenson D. M., Maher J. J., Roll F. J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987 Mar;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J. M. Induction of albumin gene transcription in hepatocytes by extracellular matrix proteins. Mol Cell Biol. 1990 Mar;10(3):1239–1243. doi: 10.1128/mcb.10.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S., Phillips S. L., Hassell J. R. Assignment of the perlecan (heparan sulfate proteoglycan) gene to mouse chromosome 4. Mamm Genome. 1991;1(4):270–272. doi: 10.1007/BF00352338. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clement B., Laurent M., Guguen-Guillouzo C., Lebeau G., Guillouzo A. Types I and IV procollagen gene expression in cultured rat hepatocytes. Coll Relat Res. 1988 Jul;8(4):349–359. doi: 10.1016/s0174-173x(88)80006-2. [DOI] [PubMed] [Google Scholar]
- Clement B., Rissel M., Peyrol S., Mazurier Y., Grimaud J. A., Guillouzo A. A procedure for light and electron microscopic intracellular immunolocalization of collagen and fibronectin in rat liver. J Histochem Cytochem. 1985 May;33(5):407–414. doi: 10.1177/33.5.3886779. [DOI] [PubMed] [Google Scholar]
- Clément B., Rescan P. Y., Baffet G., Loréal O., Lehry D., Campion J. P., Guillouzo A. Hepatocytes may produce laminin in fibrotic liver and in primary culture. Hepatology. 1988 Jul-Aug;8(4):794–803. doi: 10.1002/hep.1840080417. [DOI] [PubMed] [Google Scholar]
- Clément B., Segui-Real B., Hassell J. R., Martin G. R., Yamada Y. Identification of a cell surface-binding protein for the core protein of the basement membrane proteoglycan. J Biol Chem. 1989 Jul 25;264(21):12467–12471. [PubMed] [Google Scholar]
- Clément B., Segui-Real B., Savagner P., Kleinman H. K., Yamada Y. Hepatocyte attachment to laminin is mediated through multiple receptors. J Cell Biol. 1990 Jan;110(1):185–192. doi: 10.1083/jcb.110.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément B., Yamada Y. A Mr 80K hepatocyte surface protein(s) interacts with basement membrane components. Exp Cell Res. 1990 Apr;187(2):320–323. doi: 10.1016/0014-4827(90)90098-u. [DOI] [PubMed] [Google Scholar]
- Couchman J. R. Heterogeneous distribution of a basement membrane heparan sulfate proteoglycan in rat tissues. J Cell Biol. 1987 Oct;105(4):1901–1916. doi: 10.1083/jcb.105.4.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom M., Klein G., Mugrauer G., Fecker L., Deutzmann R., Timpl R., Ekblom P. Transient and locally restricted expression of laminin A chain mRNA by developing epithelial cells during kidney organogenesis. Cell. 1990 Jan 26;60(2):337–346. doi: 10.1016/0092-8674(90)90748-4. [DOI] [PubMed] [Google Scholar]
- Forsberg E., Paulsson M., Timpl R., Johansson S. Characterization of a laminin receptor on rat hepatocytes. J Biol Chem. 1990 Apr 15;265(11):6376–6381. [PubMed] [Google Scholar]
- Fraslin J. M., Kneip B., Vaulont S., Glaise D., Munnich A., Guguen-Guillouzo C. Dependence of hepatocyte-specific gene expression on cell-cell interactions in primary culture. EMBO J. 1985 Oct;4(10):2487–2491. doi: 10.1002/j.1460-2075.1985.tb03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts A., Geuze H. J., Slot J. W., Voss B., Schuppan D., Schellinck P., Wisse E. Immunogold localization of procollagen III, fibronectin and heparan sulfate proteoglycan on ultrathin frozen sections of the normal rat liver. Histochemistry. 1986;84(4-6):355–362. doi: 10.1007/BF00482963. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C., Clément B., Baffet G., Beaumont C., Morel-Chany E., Glaise D., Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983 Jan;143(1):47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Kimura J. H., Hascall V. C. Proteoglycan core protein families. Annu Rev Biochem. 1986;55:539–567. doi: 10.1146/annurev.bi.55.070186.002543. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Leyshon W. C., Ledbetter S. R., Tyree B., Suzuki S., Kato M., Kimata K., Kleinman H. K. Isolation of two forms of basement membrane proteoglycans. J Biol Chem. 1985 Jul 5;260(13):8098–8105. [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Sommarin Y. Proteoglycans: an overview. Methods Enzymol. 1987;144:305–319. doi: 10.1016/0076-6879(87)44185-2. [DOI] [PubMed] [Google Scholar]
- Isom I., Georgoff I., Salditt-Georgieff M., Darnell J. E., Jr Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J Cell Biol. 1987 Dec;105(6 Pt 2):2877–2885. doi: 10.1083/jcb.105.6.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker J. L., Heine U. I. Effect of adhesion factors fibronectin, laminin, and type IV collagen on spreading and growth of transformed and control rat liver epithelial cells. Cancer Res. 1987 Jul 15;47(14):3802–3807. [PubMed] [Google Scholar]
- Kanwar Y. S., Hascall V. C., Farquhar M. G. Partial characterization of newly synthesized proteoglycans isolated from the glomerular basement membrane. J Cell Biol. 1981 Aug;90(2):527–532. doi: 10.1083/jcb.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Otsu K., Ohtake K., Kimura Y., Yashiro T., Suzuki T., Akamatsu N. Concurrent changes in sinusoidal expression of laminin and affinity of hepatocytes to laminin during rat liver regeneration. Exp Cell Res. 1992 Jan;198(1):59–68. doi: 10.1016/0014-4827(92)90149-3. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Pettersson I., Hök M. Cell-surface heparan sulfate: an intercalated membrane proteoglycan. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5371–5375. doi: 10.1073/pnas.78.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. J., Brown D. M., Oegema T. R., Brenchley P. E., Anderson J. C., Dickinson M. A., Horigan E. A., Hassell J. R. Glomerular basement membrane proteoglycans are derived from a large precursor. J Cell Biol. 1988 Mar;106(3):963–970. doi: 10.1083/jcb.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Ebihara I., Killen P. D., Sasaki M., Cannon F. B., Yamada Y., Martin G. R. Genes for basement membrane proteins are coordinately expressed in differentiating F9 cells but not in normal adult murine tissues. Dev Biol. 1987 Aug;122(2):373–378. doi: 10.1016/0012-1606(87)90302-2. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Bing J. T., Kleinman H. K., Hassell J. R., Aumailley M., Martin G. R., Feldmann R. J. Localization of binding sites for laminin, heparan sulfate proteoglycan and fibronectin on basement membrane (type IV) collagen. J Mol Biol. 1986 May 5;189(1):205–216. doi: 10.1016/0022-2836(86)90391-8. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Horikoshi S., Killen P. D., Segui-Real B., Yamada Y. In situ hybridization reveals temporal and spatial changes in cellular expression of mRNA for a laminin receptor, laminin, and basement membrane (type IV) collagen in the developing kidney. J Cell Biol. 1989 Sep;109(3):1351–1362. doi: 10.1083/jcb.109.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie G. W., Inoue S., Bing J. T., Hassell J. R. Visualization of the large heparan sulfate proteoglycan from basement membrane. Am J Anat. 1988 Mar;181(3):320–326. doi: 10.1002/aja.1001810308. [DOI] [PubMed] [Google Scholar]
- Ledbetter S. R., Tyree B., Hassell J. R., Horigan E. A. Identification of the precursor protein to basement membrane heparan sulfate proteoglycans. J Biol Chem. 1985 Jul 5;260(13):8106–8113. [PubMed] [Google Scholar]
- Loreal O., Levavasseur F., Rescan P. Y., Yamada Y., Guillouzo A., Clement B. Differential expression of laminin chains in hepatic lipocytes. FEBS Lett. 1991 Sep 23;290(1-2):9–12. doi: 10.1016/0014-5793(91)81213-r. [DOI] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali M., Jaakkola P., Arvilommi A. M., Jalkanen M. Sequence of human syndecan indicates a novel gene family of integral membrane proteoglycans. J Biol Chem. 1990 Apr 25;265(12):6884–6889. [PubMed] [Google Scholar]
- Martinez-Hernandez A., Delgado F. M., Amenta P. S. The extracellular matrix in hepatic regeneration. Localization of collagen types I, III, IV, laminin, and fibronectin. Lab Invest. 1991 Feb;64(2):157–166. [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984 Jul;51(1):57–74. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Riecken E. O., Stein H. Cellular localization of laminin gene transcripts in normal and fibrotic human liver. Am J Pathol. 1989 Jun;134(6):1175–1182. [PMC free article] [PubMed] [Google Scholar]
- Noonan D. M., Fulle A., Valente P., Cai S., Horigan E., Sasaki M., Yamada Y., Hassell J. R. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991 Dec 5;266(34):22939–22947. [PubMed] [Google Scholar]
- Noonan D. M., Horigan E. A., Ledbetter S. R., Vogeli G., Sasaki M., Yamada Y., Hassell J. R. Identification of cDNA clones encoding different domains of the basement membrane heparan sulfate proteoglycan. J Biol Chem. 1988 Nov 5;263(31):16379–16387. [PubMed] [Google Scholar]
- Rescan P. Y., Clément B., Grimaud J. A., Guillois B., Strain A., Guillouzo A. Participation of hepatocytes in the production of basement membrane components in human and rat liver during the perinatal period. Cell Differ Dev. 1989 Mar;26(2):131–144. doi: 10.1016/0922-3371(89)90015-4. [DOI] [PubMed] [Google Scholar]
- Rescan P. Y., Clément B., Yamada Y., Segui-Real B., Baffet G., Guguen-Guillouzo C., Guillouzo A. Differential expression of laminin chains and receptor (LBP-32) in fetal and neoplastic hepatocytes compared to normal adult hepatocytes in vivo and in culture. Am J Pathol. 1990 Sep;137(3):701–709. [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Fujita M., Saez J. C., Choi H., Watanabe T., Hertzberg E., Rosenberg L. C., Reid L. M. Proteoglycans and glycosaminoglycans induce gap junction synthesis and function in primary liver cultures. J Cell Biol. 1987 Jul;105(1):541–551. doi: 10.1083/jcb.105.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., Kjéllen L., Unger E., Hök M., Farquhar M. G. Heparan sulfate proteoglycans are concentrated on the sinusoidal plasmalemmal domain and in intracellular organelles of hepatocytes. J Cell Biol. 1985 Mar;100(3):975–980. doi: 10.1083/jcb.100.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Dziadek M. Structure, development, and molecular pathology of basement membranes. Int Rev Exp Pathol. 1986;29:1–112. [PubMed] [Google Scholar]
- Vandenberghe Y., Ratanasavanh D., Glaise D., Guillouzo A. Influence of medium composition and culture conditions on glutathione S-transferase activity in adult rat hepatocytes during culture. In Vitro Cell Dev Biol. 1988 Apr;24(4):281–288. doi: 10.1007/BF02628828. [DOI] [PubMed] [Google Scholar]
- Weiner F. R., Giambrone M. A., Czaja M. J., Shah A., Annoni G., Takahashi S., Eghbali M., Zern M. A. Ito-cell gene expression and collagen regulation. Hepatology. 1990 Jan;11(1):111–117. doi: 10.1002/hep.1840110119. [DOI] [PubMed] [Google Scholar]