Abstract
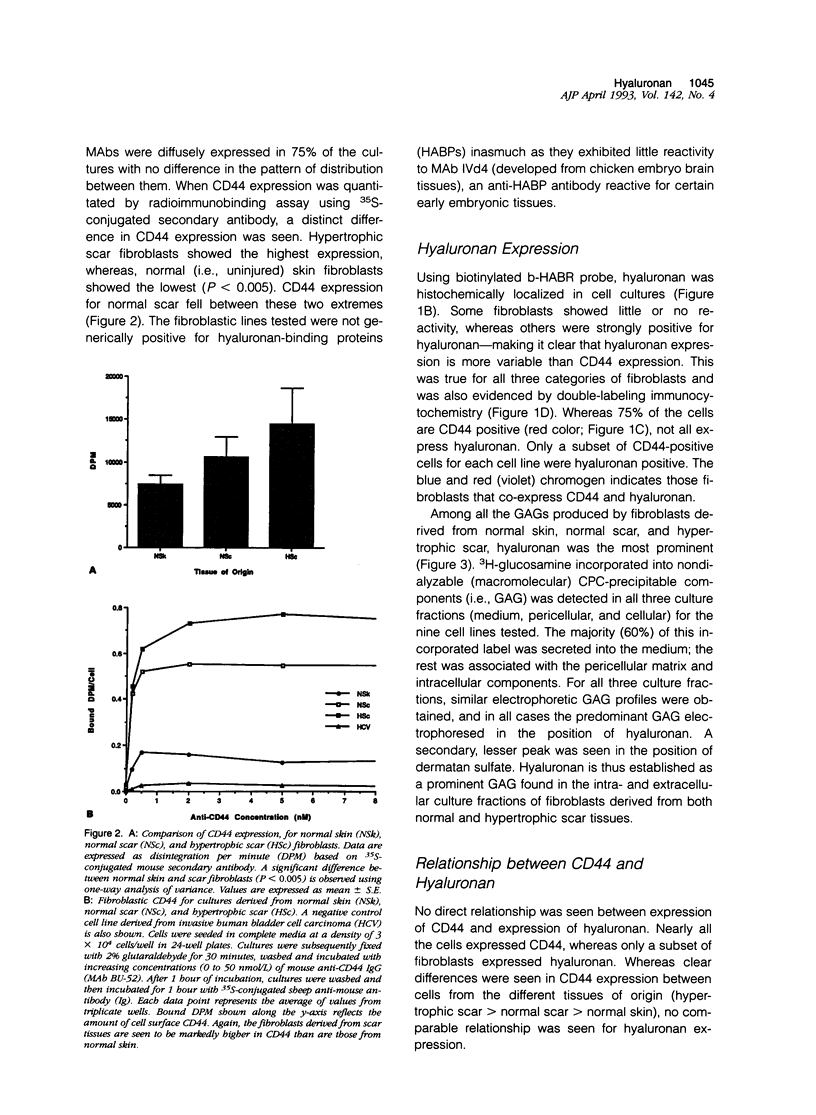
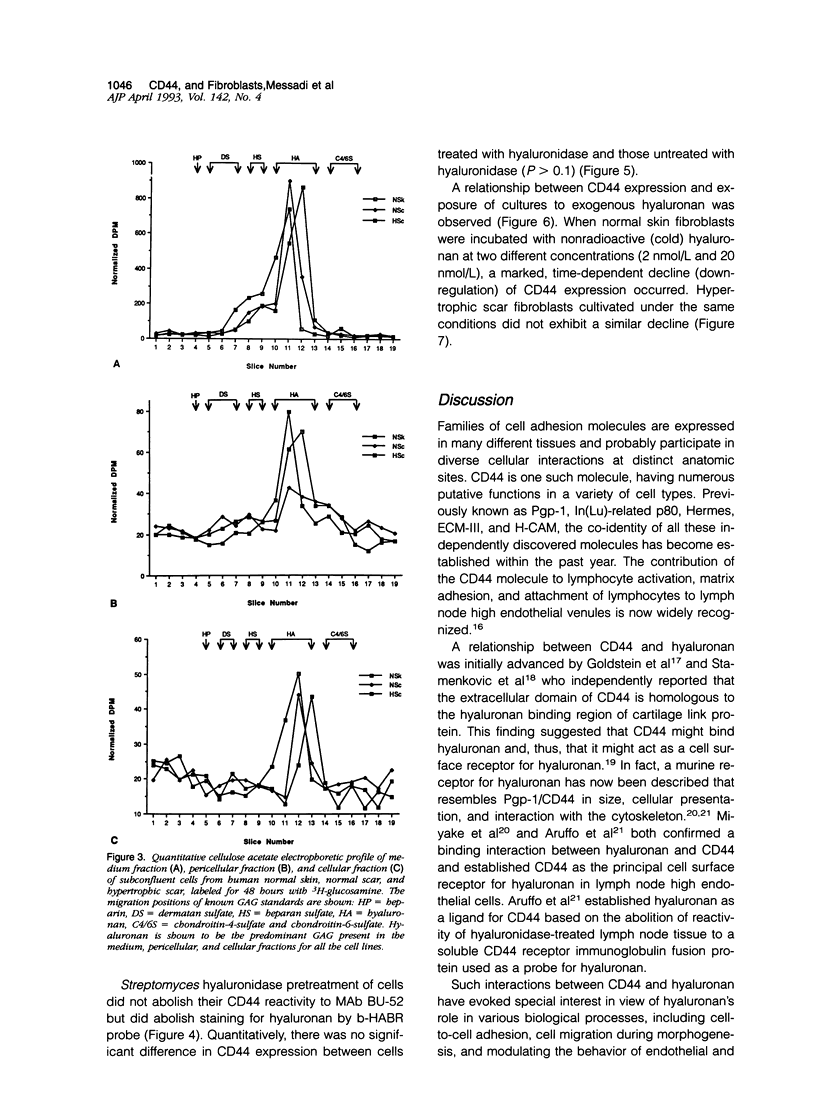
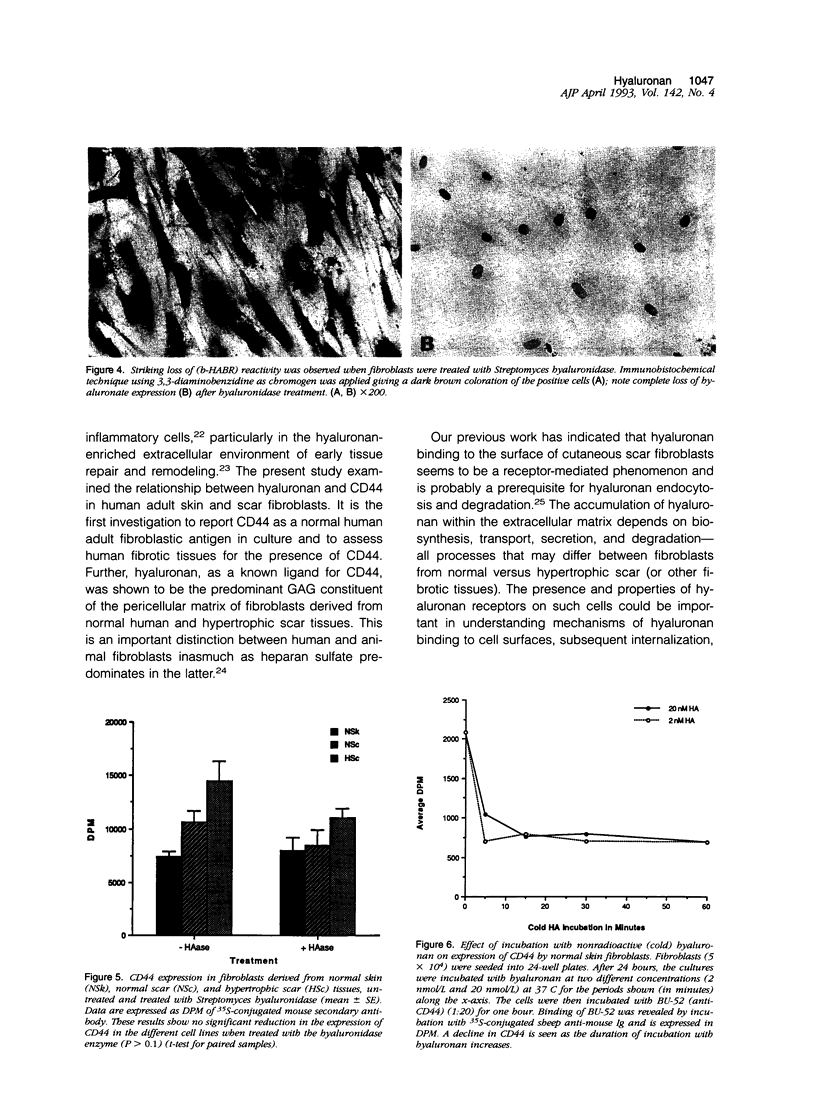
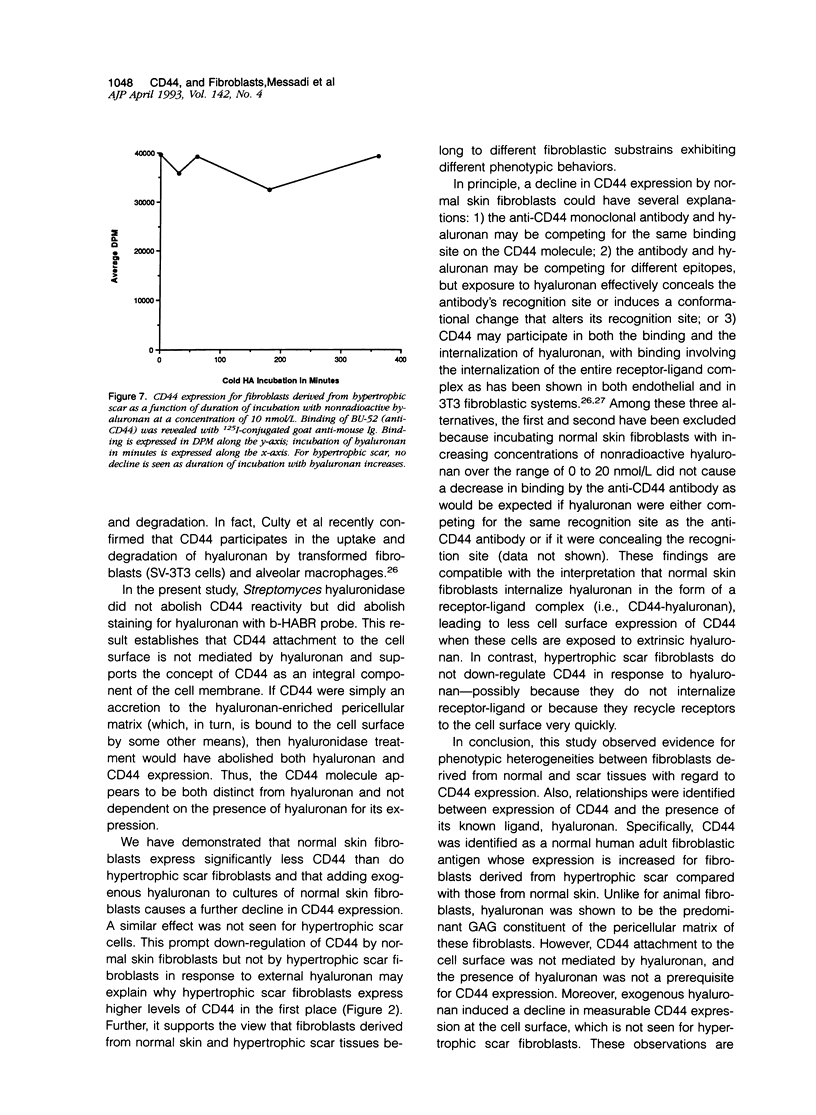
Fibrotic disorders of skin and other organs are typically associated with an abnormal accumulation of extracellular matrix. This study focuses on a matrix constituent, hyaluronan-which is known to be altered in fibrotic disorders of skin- and on CD44, a cell adhesion molecule and putative receptor for hyaluronan. Tissue samples were obtained from biopsies of human normal skin, normal cutaneous scar; and hypertrophic cutaneous scar. After culturing, cells were studied by single- and double-labeling immunohistochemistry using the two anti-CD44 monoclonal antibodies, BU-52 and J173, and a biotinylated hyaluronan binding complex probe, b-HABR. Certain cultures were pretreated with Streptomyces hyaluronidase to assess the dependency of CD44 expression on the presence of endogenous hyaluronan. CD44 expression, both in the presence and the absence of exogenous hyaluronan, was quantitated by radioimmunobinding assay. Overall glycosaminoglycan synthesis and identification of hyaluronan were accomplished by precursor incorporation assays and by quantitative cellulose acetate electrophoresis. CD44 was found to be a normal human adult fibroblastic antigen whose expression is markedly increased for hypertrophic scar fibroblasts compared with normal skin fibroblasts. Although hyaluronan was found to be the predominant glycosaminoglycan constituent of the pericellular matrix for these fibroblasts, CD44 attachment to the cell surface is neither mediated by hyaluronan nor is the presence of hyaluronan a prerequisite for CD44 expression. Exogenous hyaluronan induced a decline in measurable CD44 expression for normal skin fibroblasts but not for hypertrophic scar fibroblasts. These observations are compatible with current understanding of the way cells manage the hyaluronan economy of the extracellular matrix and emphasize phenotypic heterogeneities between fibroblasts derived from normal versus scar tissues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Banerjee S. D., Toole B. P. Monoclonal antibody to chick embryo hyaluronan-binding protein: changes in distribution of binding protein during early brain development. Dev Biol. 1991 Jul;146(1):186–197. doi: 10.1016/0012-1606(91)90459-g. [DOI] [PubMed] [Google Scholar]
- Bertolami C. N., Berg S., Messadi D. V. Binding and internalization of hyaluronate by human cutaneous fibroblasts. Matrix. 1992 Feb;12(1):11–21. doi: 10.1016/s0934-8832(11)80100-9. [DOI] [PubMed] [Google Scholar]
- Botstein G. R., Sherer G. K., Leroy E. C. Fibroblast selection in scleroderma. An alternative model of fibrosis. Arthritis Rheum. 1982 Feb;25(2):189–195. doi: 10.1002/art.1780250212. [DOI] [PubMed] [Google Scholar]
- Bronson R. E., Argenta J. G., Siebert E. P., Bertolami C. N. Distinctive fibroblastic subpopulations in skin and oral mucosa demonstrated by differences in glycosaminoglycan content. In Vitro Cell Dev Biol. 1988 Nov;24(11):1121–1126. doi: 10.1007/BF02620814. [DOI] [PubMed] [Google Scholar]
- Bronson R. E., Bertolami C. N., Siebert E. P. Modulation of fibroblast growth and glycosaminoglycan synthesis by interleukin-1. Coll Relat Res. 1987 Oct;7(5):323–332. doi: 10.1016/s0174-173x(87)80025-0. [DOI] [PubMed] [Google Scholar]
- Cappelletti R., Del Rosso M., Chiarugi V. P. A new electrophoretic method for the complete separation of all known animal glycosaminoglycans in a monodimensional run. Anal Biochem. 1979 Nov 1;99(2):311–315. doi: 10.1016/s0003-2697(79)80012-3. [DOI] [PubMed] [Google Scholar]
- Cappelletti R., Del Rosso M., Chiarugi V. P. Rapid multisample separation of the five most widespread animal glycosaminoglycans. Anal Biochem. 1979 Feb;93(1):37–40. [PubMed] [Google Scholar]
- Culty M., Miyake K., Kincade P. W., Sikorski E., Butcher E. C., Underhill C., Silorski E. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990 Dec;111(6 Pt 1):2765–2774. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culty M., Nguyen H. A., Underhill C. B. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992 Feb;116(4):1055–1062. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann R. F., Cohen I. K., McCoy B. J. Growth kinetics and collagen synthesis of normal skin, normal scar and keloid fibroblasts in vitro. J Cell Physiol. 1979 Feb;98(2):341–346. doi: 10.1002/jcp.1040980210. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Lord R. H., Colby A. J., Diamantstein T., Rickles F. R., Dijkstra C., Hogg N., Tilney N. L. Identification of IL 2R+ T cells and macrophages within rejecting rat cardiac allografts, and comparison of the effects of treatment with anti-IL 2R monoclonal antibody or cyclosporin. J Immunol. 1987 Jan 1;138(1):164–170. [PubMed] [Google Scholar]
- Haynes B. F., Telen M. J., Hale L. P., Denning S. M. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989 Dec;10(12):423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Fraser J. R. Hyaluronan. FASEB J. 1992 Apr;6(7):2397–2404. [PubMed] [Google Scholar]
- Lesley J., Schulte R., Hyman R. Binding of hyaluronic acid to lymphoid cell lines is inhibited by monoclonal antibodies against Pgp-1. Exp Cell Res. 1990 Apr;187(2):224–233. doi: 10.1016/0014-4827(90)90085-o. [DOI] [PubMed] [Google Scholar]
- McGary C. T., Raja R. H., Weigel P. H. Endocytosis of hyaluronic acid by rat liver endothelial cells. Evidence for receptor recycling. Biochem J. 1989 Feb 1;257(3):875–884. doi: 10.1042/bj2570875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messadi D. V., Pober J. S., Murphy G. F. Effects of recombinant gamma-interferon on HLA-DR and DQ expression by skin cells in short-term organ culture. Lab Invest. 1988 Jan;58(1):61–67. [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell W. B., Cohen I. K., Ehrlich H. P. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989 Nov;84(5):827–837. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]




