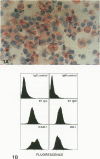
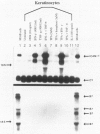
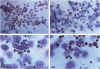
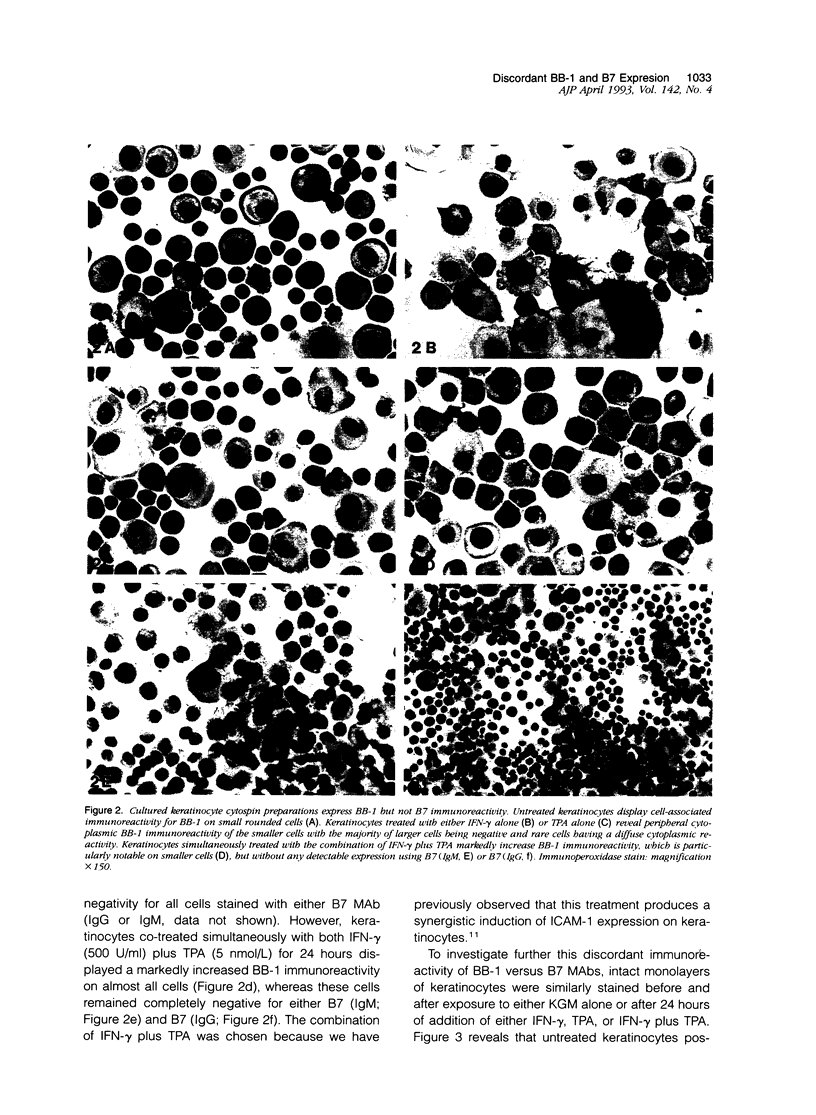
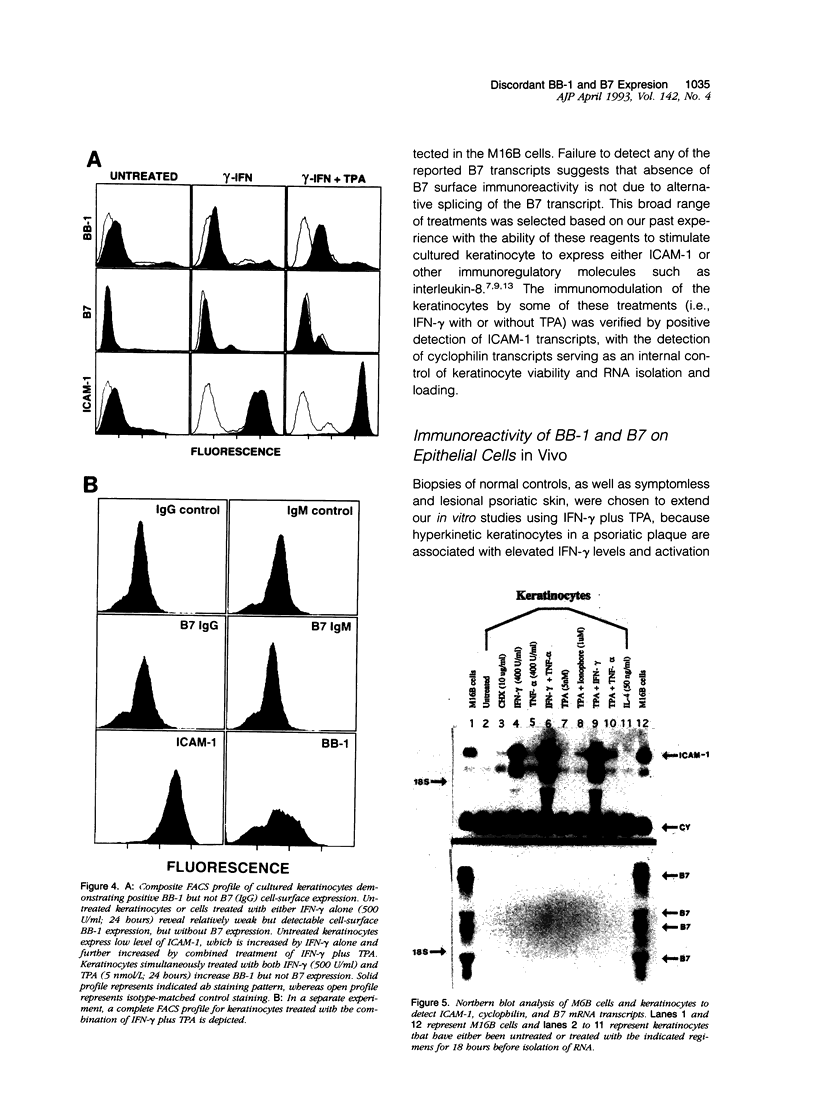
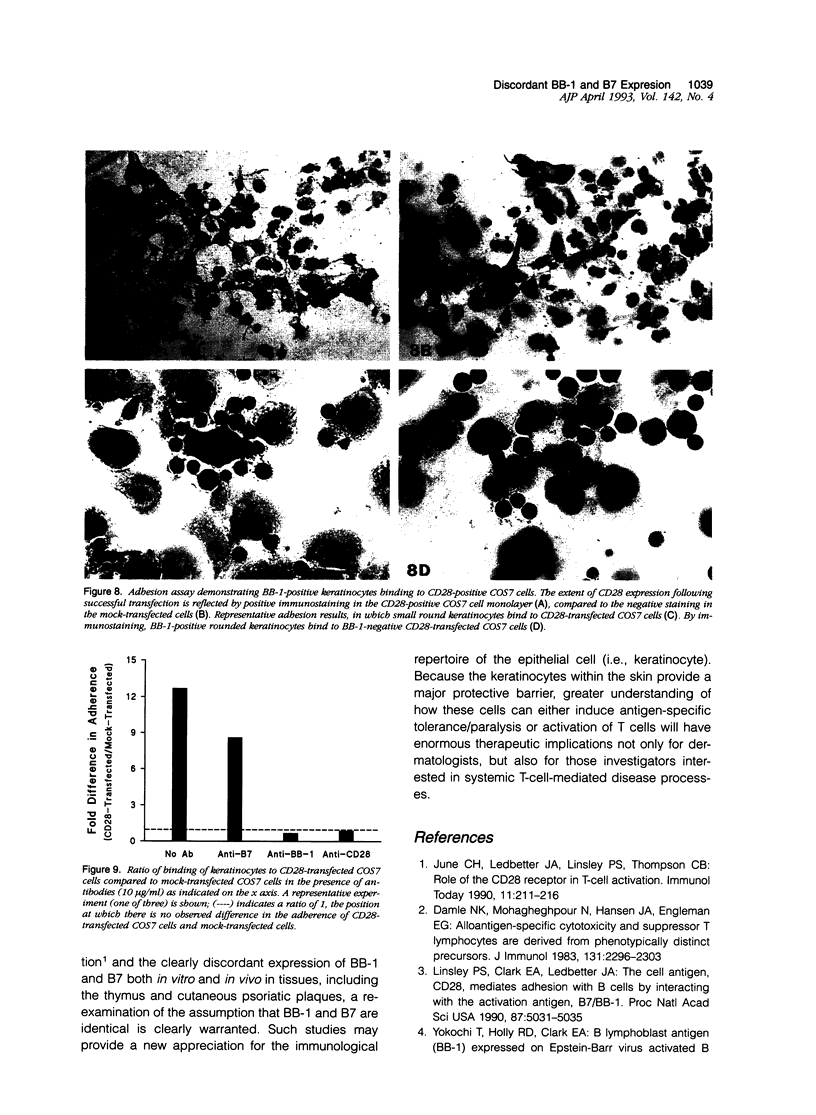
Abstract
The process for optimal T-cell activation requires not only engagement of the T-cell receptor/CD3 complex, but also the delivery of additional co-stimulatory signals that synergize with the primary response mediated through the T-cell receptor. Thus, the regulated expression of ligands for such co-stimulatory molecules can be critical in determining whether a cell can effectively activate T cells following the presentation of a foreign antigen. The CD28 antigen has recently been shown to mediate such co-stimulatory signals by interacting with the B7/BB-1 molecule expressed on activated B cells and monocytes. We show in this study that activated keratinocytes, both in vitro and in vivo display a discordance in expression between B7 and BB-1 based on differential monoclonal antibody (MAb) reactivity. Activated keratinocytes in vitro, as well as psoriatic keratinocytes and epithelial cells in the thymus, are reactive with the BB-1 MAb but not anti-B7 MAbs. These BB-1 positive cells fail to express detectable B7 messenger RNA by Northern blot analysis. Furthermore, keratinocytes bind specifically to CD28-transfected COS7 cells, and this binding is inhibited by anti-CD28 and anti-BB-1 but not B7 MAbs. These studies suggest: 1) that the MAb against BB-1 binds a functional epitope on a molecule distinct from B7 as detected on activated keratinocytes in vitro and in vivo and 2) that keratinocytes in skin and epithelial cells in thymus can express cell-surface molecules that might mediate T-cell co-stimulation via CD28.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal V., McIndoe A., Denton G., Hudson D., Lombardi G., Lamb J., Lechler R. Antigen presentation by keratinocytes induces tolerance in human T cells. Eur J Immunol. 1990 Sep;20(9):1893–1897. doi: 10.1002/eji.1830200904. [DOI] [PubMed] [Google Scholar]
- Barker J. N., Karabin G. D., Stoof T. J., Sarma V. J., Dixit V. M., Nickoloff B. J. Detection of interferon-gamma mRNA in psoriatic epidermis by polymerase chain reaction. J Dermatol Sci. 1991 Mar;2(2):106–111. doi: 10.1016/0923-1811(91)90019-t. [DOI] [PubMed] [Google Scholar]
- Barker J. N., Mitra R. S., Griffiths C. E., Dixit V. M., Nickoloff B. J. Keratinocytes as initiators of inflammation. Lancet. 1991 Jan 26;337(8735):211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Barker J. N., Sarma V., Mitra R. S., Dixit V. M., Nickoloff B. J. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J Clin Invest. 1990 Feb;85(2):605–608. doi: 10.1172/JCI114481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Mohagheghpour N., Hansen J. A., Engleman E. G. Alloantigen-specific cytotoxic and suppressor T lymphocytes are derived from phenotypically distinct precursors. J Immunol. 1983 Nov;131(5):2296–2300. [PubMed] [Google Scholar]
- Freedman A. S., Freeman G. J., Rhynhart K., Nadler L. M. Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991 Oct 15;137(2):429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G., Horowitz J. C., Daley J., Nadler L. M. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol. 1987 Nov 15;139(10):3260–3267. [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Segil J. M., Lee G., Whitman J. F., Nadler L. M. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989 Oct 15;143(8):2714–2722. [PubMed] [Google Scholar]
- Gaspari A. A., Jenkins M. K., Katz S. I. Class II MHC-bearing keratinocytes induce antigen-specific unresponsiveness in hapten-specific Th1 clones. J Immunol. 1988 Oct 1;141(7):2216–2220. [PubMed] [Google Scholar]
- Griffiths C. E., Esmann J., Fisher G. J., Voorhees J. J., Nickoloff B. J. Differential modulation of keratinocyte intercellular adhesion molecule-I expression by gamma interferon and phorbol ester: evidence for involvement of protein kinase C signal transduction. Br J Dermatol. 1990 Mar;122(3):333–342. doi: 10.1111/j.1365-2133.1990.tb08281.x. [DOI] [PubMed] [Google Scholar]
- Griffiths C. E., Voorhees J. J., Nickoloff B. J. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989 Apr;20(4):617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Shimizu Y., Barker J. N., Karabin G., Stoof T., Stoolman L. M. HUT 78 T cells bind to noncytokine-stimulated keratinocytes using a non-CD18-dependent adhesion pathway. Am J Pathol. 1992 Jun;140(6):1365–1374. [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J. The cytokine network in psoriasis. Arch Dermatol. 1991 Jun;127(6):871–884. [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Paine R., 3rd, Schieven G. L., Thompson G. B., Ledbetter J. A. Signal transduction via CD4, CD8, and CD28 in mature and immature thymocytes. Implications for thymic selection. J Immunol. 1991 Mar 1;146(5):1428–1436. [PubMed] [Google Scholar]
- Vollger L. W., Tuck D. T., Springer T. A., Haynes B. F., Singer K. H. Thymocyte binding to human thymic epithelial cells is inhibited by monoclonal antibodies to CD-2 and LFA-3 antigens. J Immunol. 1987 Jan 15;138(2):358–363. [PubMed] [Google Scholar]