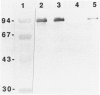
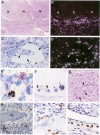
Abstract
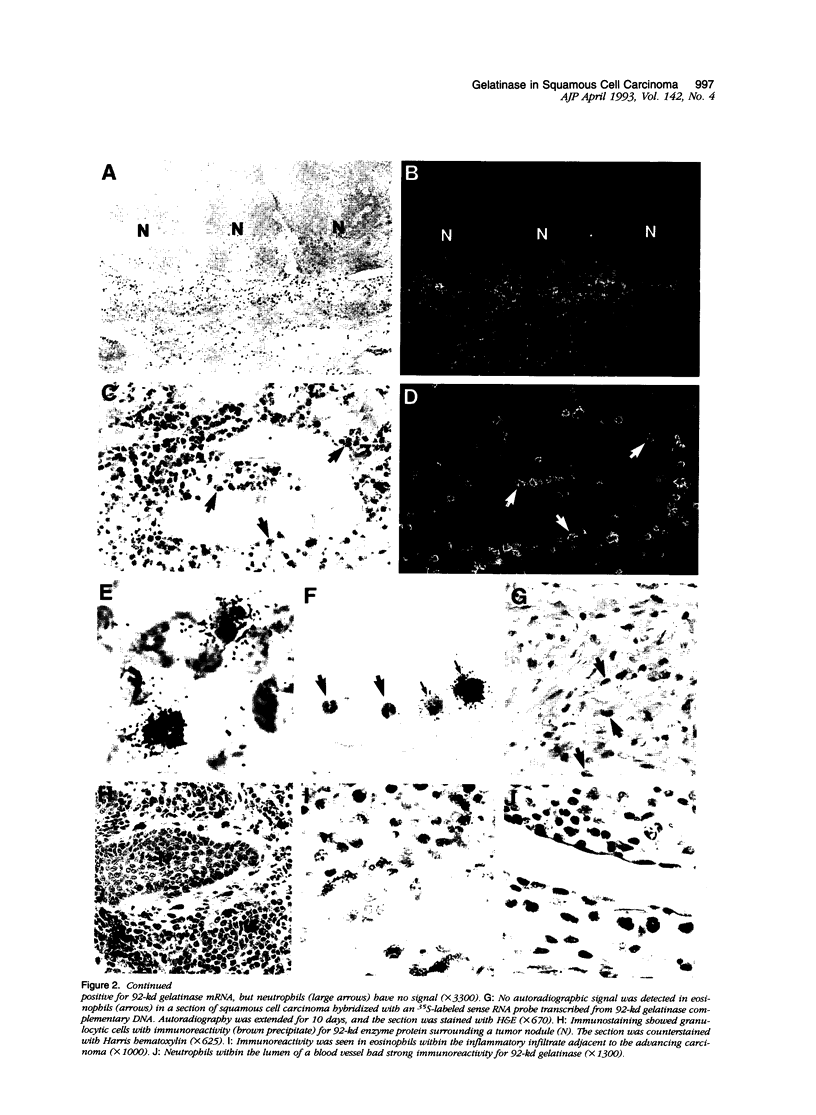
Tumor invasion and metastasis are assisted by multiple proteinases that degrade basement membrane and stromal matrix components. We used in situ hybridization with 35S-labeled RNA probes and immunohistochemistry to localize cellular sites of 92-kd gelatinase production in sections of invasive squamous cell carcinoma. Signal for enzyme messenger RNA was detected only in numerous eosinophils that surrounded the tumor nodules, and immunohistochemical staining verified the presence of enzyme protein in these granulocytes and also revealed strong reactivity in neutrophils. No resident or other migratory cell type was positive for gelatinase messenger RNA or protein, and no signal was detected by either assay in samples of healthy skin. These data indicate that eosinophils have the capacity to synthesize actively 92-kd gelatinase, whereas neutrophils store and probably release the enzyme on demand. Because of the capacity of 92-kd gelatinase to degrade both basement membrane and interstitial extracellular matrix molecules, the expression, delivery, and secretion of this metalloproteinase by granulocytes may be critical for tumor invasion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard E. J., Muschel R. J., Hughes E. N. Mr 92,000 gelatinase release correlates with the metastatic phenotype in transformed rat embryo cells. Cancer Res. 1990 Jul 1;50(13):3872–3877. [PubMed] [Google Scholar]
- Claudatus J. C., Jr, d'Ovidio R., Lospalluti M., Meneghini C. L. Skin tumors and reactive cellular infiltrate: further studies. Acta Derm Venereol. 1986;66(1):29–34. [PubMed] [Google Scholar]
- Goldberg G. I., Eisen A. Z. Extracellular matrix metalloproteinases in tumor invasion and metastasis. Cancer Treat Res. 1991;53:421–440. doi: 10.1007/978-1-4615-3940-7_20. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S. Expression of 92 kDa phagocyte gelatinase by inflammatory and connective tissue cells. Matrix Suppl. 1992;1:51–57. [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Hibbs M. S., Hoidal J. R., Kang A. H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987 Dec;80(6):1644–1650. doi: 10.1172/JCI113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacraz S., Nicod L., Galve-de Rochemonteix B., Baumberger C., Dayer J. M., Welgus H. G. Suppression of metalloproteinase biosynthesis in human alveolar macrophages by interleukin-4. J Clin Invest. 1992 Aug;90(2):382–388. doi: 10.1172/JCI115872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Lenihan D. J., Malone B., Roddy L. L., Wasserman S. I. Increased biosynthesis of platelet-activating factor in activated human eosinophils. J Biol Chem. 1984 May 10;259(9):5526–5530. [PubMed] [Google Scholar]
- Liotta L. A., Wewer U., Rao N. C., Schiffmann E., Stracke M., Guirguis R., Thorgeirsson U., Muschel R., Sobel M. Biochemical mechanisms of tumor invasion and metastases. Adv Exp Med Biol. 1988;233:161–169. doi: 10.1007/978-1-4899-5037-6_18. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Ward R., Hembry R. M., Reynolds J. J., Kühn K., Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989 Mar 1;258(2):463–472. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Morikawa K., Fabra A., Bucana C. D., Fidler I. J. Influence of organ environment on extracellular matrix degradative activity and metastasis of human colon carcinoma cells. J Natl Cancer Inst. 1990 Dec 19;82(24):1890–1898. doi: 10.1093/jnci/82.24.1890. [DOI] [PubMed] [Google Scholar]
- Prosser I. W., Stenmark K. R., Suthar M., Crouch E. C., Mecham R. P., Parks W. C. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989 Dec;135(6):1073–1088. [PMC free article] [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Huhtala P., Hurskainen T., Danø K., Tryggvason K. Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenases in human skin cancers by in situ hybridization. Cancer Res. 1992 Mar 1;52(5):1336–1341. [PubMed] [Google Scholar]
- Schleimer R. P., Sterbinsky S. A., Kaiser J., Bickel C. A., Klunk D. A., Tomioka K., Newman W., Luscinskas F. W., Gimbrone M. A., Jr, McIntyre B. W. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992 Feb 15;148(4):1086–1092. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Sudbeck B. D., Eisen A. Z., Welgus H. G., Parks W. C. Expression of 92-kDa type IV collagenase mRNA by eosinophils associated with basal cell carcinoma. J Invest Dermatol. 1992 Oct;99(4):497–503. doi: 10.1111/1523-1747.ep12616171. [DOI] [PubMed] [Google Scholar]
- Todd R., Chou M. Y., Matossian K., Gallagher G. T., Donoff R. B., Wong D. T. Cellular sources of transforming growth factor-alpha in human oral cancer. J Dent Res. 1991 May;70(5):917–923. doi: 10.1177/00220345910700051101. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Wrenn D. S., Parks W. C., Whitehouse L. A., Crouch E. C., Kucich U., Rosenbloom J., Mecham R. P. Identification of multiple tropoelastins secreted by bovine cells. J Biol Chem. 1987 Feb 15;262(5):2244–2249. [PubMed] [Google Scholar]