Abstract
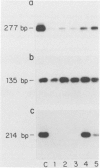
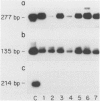
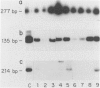
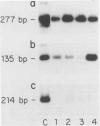
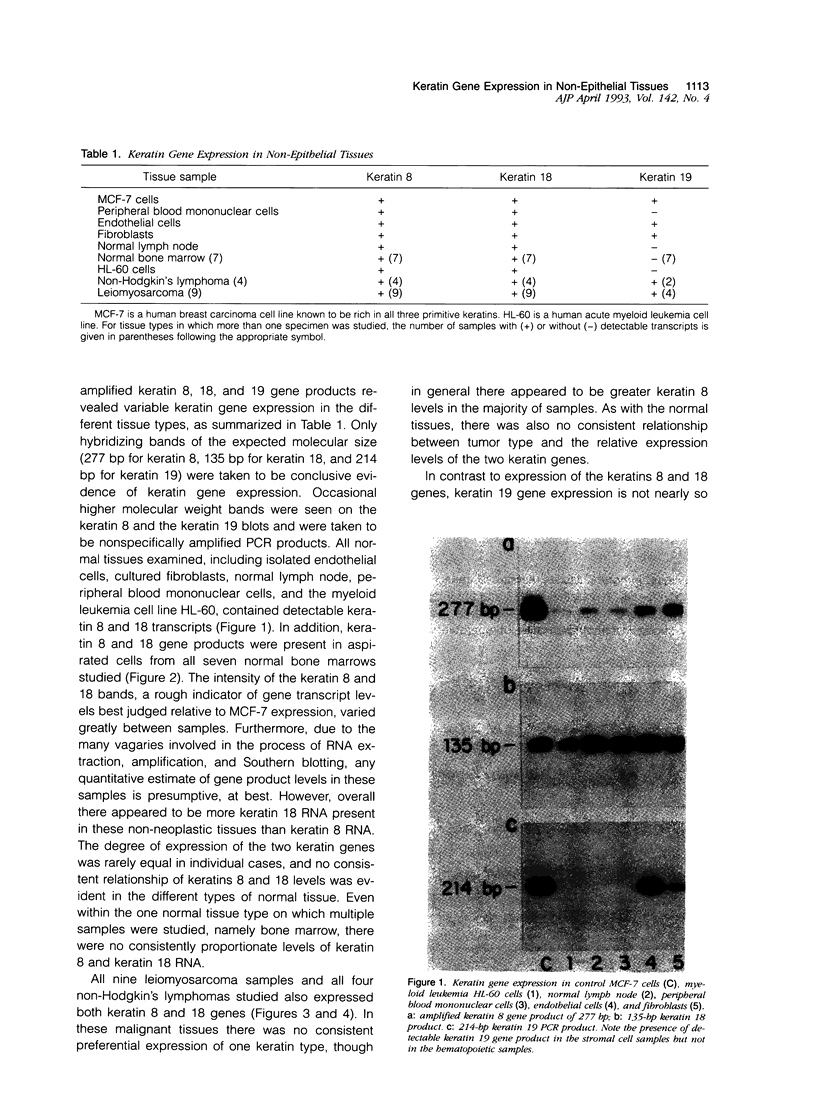
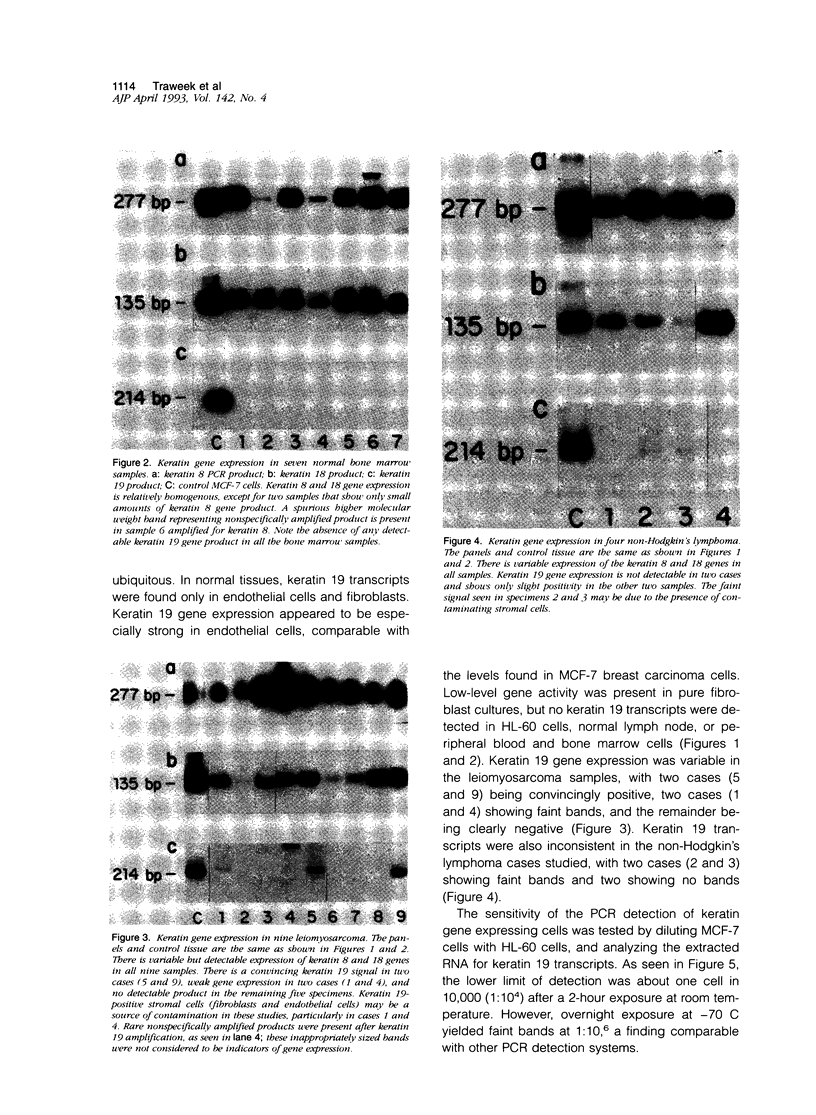
Keratin filament are characteristically present in epithelial cells and tumors, but have also been detected in many normal and neoplastic non-epithelial cell types using immunohistochemical techniques. To investigate the validity of this seemingly aberrant protein expression, we applied the highly sensitive polymerase chain reaction (PCR) technique to study keratin gene expression in a variety of non-epithelial tissues. Total RNA was extracted from nine samples of leiomyosarcoma, four non-Hodgkin's lymphoma, seven normal bone marrows, normal lymph node, normal peripheral blood cells, freshly isolated and cultured endothelial cells, cultured skin fibroblasts, and the myeloid leukemia cell line HL-60. Amplification primers and probes for the three most primitive keratin types (8, 18, and 19) were synthesized using published gene sequences. RNA from the breast carcinoma cell line MCF-7, known to be rich in all three keratins, was used as positive control. Concurrently run actin primers were used to confirm RNA integrity. After an initial cycle with reverse transcriptase, PCR amplification was performed for 30 cycles. Southern blots of the PCR products showed variably intense bands corresponding to keratin 8 and 18 gene products in all samples, offering conclusive evidence of keratin gene expression in cells of both stromal and hematopoietic derivation. However, keratin 19 gene transcription was not nearly so ubiquitous, being detected in normal fibroblasts and endothelial cells, two of four non-Hodgkin's lymphoma and four of nine leiomyosarcoma, but not in normal lymph node, peripheral blood cells, HL-60 cells, or any of the seven normal bone marrows examined. Dilutional experiments showed PCR to be highly sensitive in the detection of keratin 19 gene expression, capable of registering one MCF-7 cell in 10(6) HL-60 cells. These studies show that variable levels of keratin 8 and 18 gene expression may be detected by PCR in a wide variety of non-epithelial tissues, supporting previous immunohistochemical and phylogenetic studies. However, keratin 19 gene expression appears to be more restricted and was not evident in any hematopoietic cells devoid of contaminating stromal elements. These findings suggest a role for PCR in the detection of epithelial micrometastasis in certain sites, particularly bone marrow.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader B. L., Jahn L., Franke W. W. Low level expression of cytokeratins 8, 18 and 19 in vascular smooth muscle cells of human umbilical cord and in cultured cells derived therefrom, with an analysis of the chromosomal locus containing the cytokeratin 19 gene. Eur J Cell Biol. 1988 Dec;47(2):300–319. [PubMed] [Google Scholar]
- Battifora H. Cytokeratins in plasmacytomas. Histopathology. 1989 Sep;15(3):321–322. doi: 10.1111/j.1365-2559.1989.tb03097.x. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Theaker J. M., Banks P. M., Gatter K. C., Mason D. Y. Cytokeratin expression in smooth muscle and smooth muscle tumours. Histopathology. 1987 May;11(5):477–486. doi: 10.1111/j.1365-2559.1987.tb02656.x. [DOI] [PubMed] [Google Scholar]
- Chase D. R., Enzinger F. M., Weiss S. W., Langloss J. M. Keratin in epithelioid sarcoma. An immunohistochemical study. Am J Surg Pathol. 1984 Jun;8(6):435–441. doi: 10.1097/00000478-198406000-00004. [DOI] [PubMed] [Google Scholar]
- Crescenzi M., Seto M., Herzig G. P., Weiss P. D., Griffith R. C., Korsmeyer S. J. Thermostable DNA polymerase chain amplification of t(14;18) chromosome breakpoints and detection of minimal residual disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4869–4873. doi: 10.1073/pnas.85.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 1989 Aug;3(10):2141–2150. doi: 10.1096/fasebj.3.10.2666230. [DOI] [PubMed] [Google Scholar]
- Eusebi V., Carcangiu M. L., Dina R., Rosai J. Keratin-positive epithelioid angiosarcoma of thyroid. A report of four cases. Am J Surg Pathol. 1990 Aug;14(8):737–747. doi: 10.1097/00000478-199008000-00004. [DOI] [PubMed] [Google Scholar]
- Farhood A. I., Abrams J. Immunohistochemistry of endometrial stromal sarcoma. Hum Pathol. 1991 Mar;22(3):224–230. doi: 10.1016/0046-8177(91)90154-h. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Moll R. Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation. 1987;36(2):145–163. doi: 10.1111/j.1432-0436.1987.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Intermediate-sized filaments of human endothelial cells. J Cell Biol. 1979 Jun;81(3):570–580. doi: 10.1083/jcb.81.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Boyd H. C., Chang Y., Ferguson M., Reichler B., Tippens D. Smooth muscle cells can express cytokeratins of "simple" epithelium. Immunocytochemical and biochemical studies in vitro and in vivo. Am J Pathol. 1988 Aug;132(2):223–232. [PMC free article] [PubMed] [Google Scholar]
- Gray M. H., Rosenberg A. E., Dickersin G. R., Bhan A. K. Cytokeratin expression in epithelioid vascular neoplasms. Hum Pathol. 1990 Feb;21(2):212–217. doi: 10.1016/0046-8177(90)90131-n. [DOI] [PubMed] [Google Scholar]
- Gusterson B. A. Is keratin present in smooth muscle? Histopathology. 1987 May;11(5):549–552. doi: 10.1111/j.1365-2559.1987.tb02663.x. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Chu Y. W., Seftor R. E., Nagle R. B., McDaniel K. M., Leong S. P., Yohem K. H., Leibovitz A. M., Meyskens F. L., Jr Coexpression of vimentin and keratins by human melanoma tumor cells: correlation with invasive and metastatic potential. J Natl Cancer Inst. 1992 Feb 5;84(3):165–174. doi: 10.1093/jnci/84.3.165. [DOI] [PubMed] [Google Scholar]
- Jahn L., Fouquet B., Rohe K., Franke W. W. Cytokeratins in certain endothelial and smooth muscle cells of two taxonomically distant vertebrate species, Xenopus laevis and man. Differentiation. 1987;36(3):234–254. doi: 10.1111/j.1432-0436.1987.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Krauss S., Franke W. W. Organization and sequence of the human gene encoding cytokeratin 8. Gene. 1990 Feb 14;86(2):241–249. doi: 10.1016/0378-1119(90)90285-y. [DOI] [PubMed] [Google Scholar]
- Markl J., Franke W. W. Localization of cytokeratins in tissues of the rainbow trout: fundamental differences in expression pattern between fish and higher vertebrates. Differentiation. 1988 Dec;39(2):97–122. doi: 10.1111/j.1432-0436.1988.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Franssila K. Immunohistochemical spectrum of malignant melanoma. The common presence of keratins. Lab Invest. 1989 Dec;61(6):623–628. [PubMed] [Google Scholar]
- Miettinen M. Immunoreactivity for cytokeratin and epithelial membrane antigen in leiomyosarcoma. Arch Pathol Lab Med. 1988 Jun;112(6):637–640. [PubMed] [Google Scholar]
- Miettinen M., Rapola J. Immunohistochemical spectrum of rhabdomyosarcoma and rhabdomyosarcoma-like tumors. Expression of cytokeratin and the 68-kD neurofilament protein. Am J Surg Pathol. 1989 Feb;13(2):120–132. doi: 10.1097/00000478-198902000-00005. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Soini Y. Malignant fibrous histiocytoma. Heterogeneous patterns of intermediate filament proteins by immunohistochemistry. Arch Pathol Lab Med. 1989 Dec;113(12):1363–1366. [PubMed] [Google Scholar]
- Miettinen M., Virtanen I. Synovial sarcoma--a misnomer. Am J Pathol. 1984 Oct;117(1):18–25. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Lee I., Gould V. E., Berndt R., Roessner A., Franke W. W. Immunocytochemical analysis of Ewing's tumors. Patterns of expression of intermediate filaments and desmosomal proteins indicate cell type heterogeneity and pluripotential differentiation. Am J Pathol. 1987 May;127(2):288–304. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Löwe A., Laufer J., Franke W. W. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992 Feb;140(2):427–447. [PMC free article] [PubMed] [Google Scholar]
- Nagle R. B. Intermediate filaments: a review of the basic biology. Am J Surg Pathol. 1988;12 (Suppl 1):4–16. [PubMed] [Google Scholar]
- Nagle R. B., McDaniel K. M., Clark V. A., Payne C. M. The use of antikeratin antibodies in the diagnosis of human neoplasms. Am J Clin Pathol. 1983 Apr;79(4):458–466. doi: 10.1093/ajcp/79.4.458. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Thomas J. A., Isaacson P. G. Cytokeratin-specific monoclonal antibodies are reactive with tumours of smooth muscle derivation. An immunocytochemical and biochemical study using antibodies to intermediate filament cytoskeletal proteins. Histopathology. 1987 May;11(5):487–499. doi: 10.1111/j.1365-2559.1987.tb02657.x. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Millán J. L., Ceceña G. Comparison of mouse and human keratin 18: a component of intermediate filaments expressed prior to implantation. Differentiation. 1986;33(1):61–68. doi: 10.1111/j.1432-0436.1986.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Sewell H. F., Thompson W. D., King D. J. IgD myeloma/immunoblastic lymphoma cells expressing cytokeratin. Br J Cancer. 1986 May;53(5):695–696. doi: 10.1038/bjc.1986.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneyoshi M., Daimaru Y., Hashimoto H., Enjoji M. Malignant soft tissue neoplasms with the histologic features of renal rhabdoid tumors: an ultrastructural and immunohistochemical study. Hum Pathol. 1985 Dec;16(12):1235–1242. doi: 10.1016/s0046-8177(85)80036-8. [DOI] [PubMed] [Google Scholar]
- Turley H., Pulford K. A., Gatter K. C., Mason D. Y. Biochemical evidence that cytokeratins are present in smooth muscle. Br J Exp Pathol. 1988 Jun;69(3):433–440. [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon A. C., Norton A. J., Isaacson P. G. Immunoreactive cytokeratins in plasmacytomas. Histopathology. 1989 Feb;14(2):141–150. doi: 10.1111/j.1365-2559.1989.tb02124.x. [DOI] [PubMed] [Google Scholar]
- van Haelst U. J., Pruszczynski M., ten Cate L. N., Mravunac M. Ultrastructural and immunohistochemical study of epithelioid hemangioendothelioma of bone: coexpression of epithelial and endothelial markers. Ultrastruct Pathol. 1990 Mar-Apr;14(2):141–149. [PubMed] [Google Scholar]