Abstract
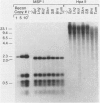
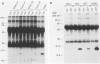
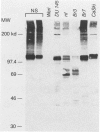
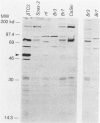
The effect of the human papillomavirus (HPV) oncogenes E6 and E7 was examined in transgenic mice with a construct containing the human beta-actin promoter regulating HPV16 E6 and E7 open reading frames. In the sole line of mice that transmitted the transgene, neuroepithelial tumors appeared at 2.5 months of life, and by 10 months, 87 of 122 (71%) of the animals were dead from brain tumors. The most frequent type of tumor (74%) was an anaplastic neuroepithelial tumor associated with the ependyma of the third and fourth ventricles, which locally invaded adjacent brain tissue and spread for considerable distances along the ventricular surface. The other two types of tumor were well-differentiated choroid plexus carcinomas (26%) and rare pituitary carcinomas (8.7%). HPV16 E6 RNA and E7 oncoprotein expression were demonstrated in tumor tissue and primary cell lines derived from the tumors. Examination of two tumor suppressor gene products, the retinoblastoma protein and p53, known to bind to HPV16 E7 and E6 oncoproteins, respectively, showed both were expressed in the primary tumor cell lines. These data support a causative role for the HPV oncoproteins in epithelial carcinogenesis.
Full text
PDF




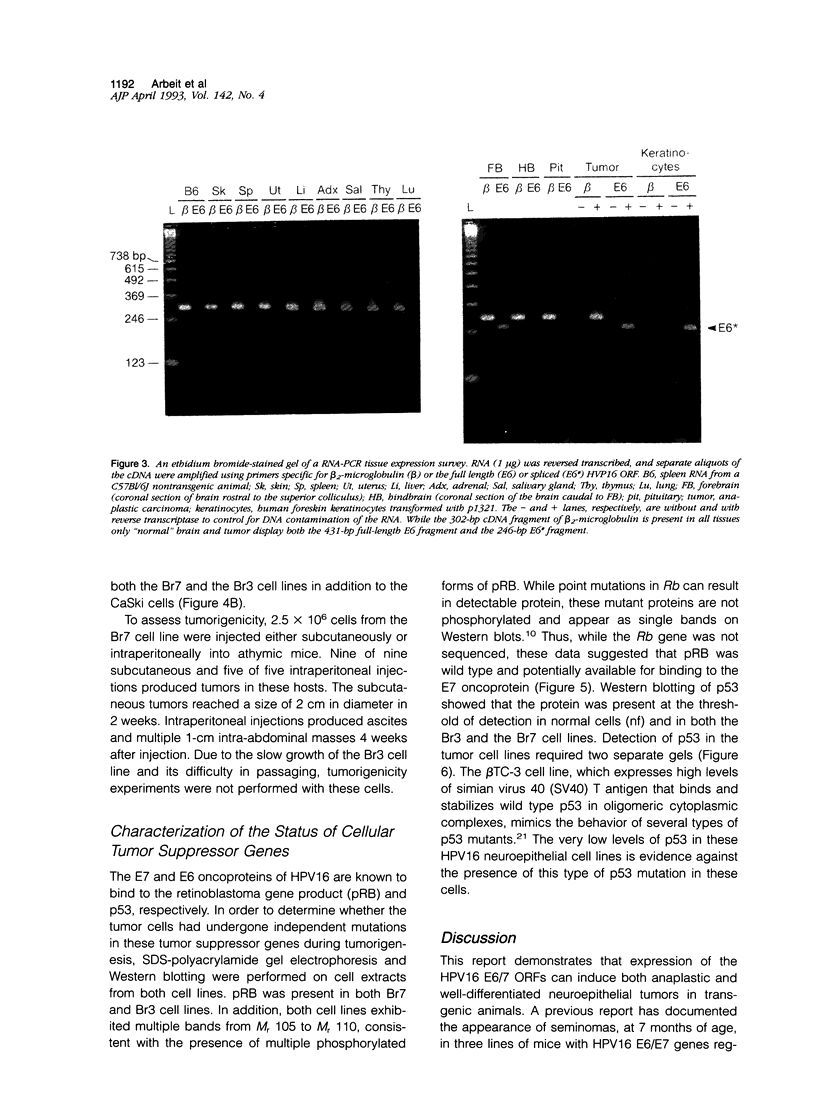
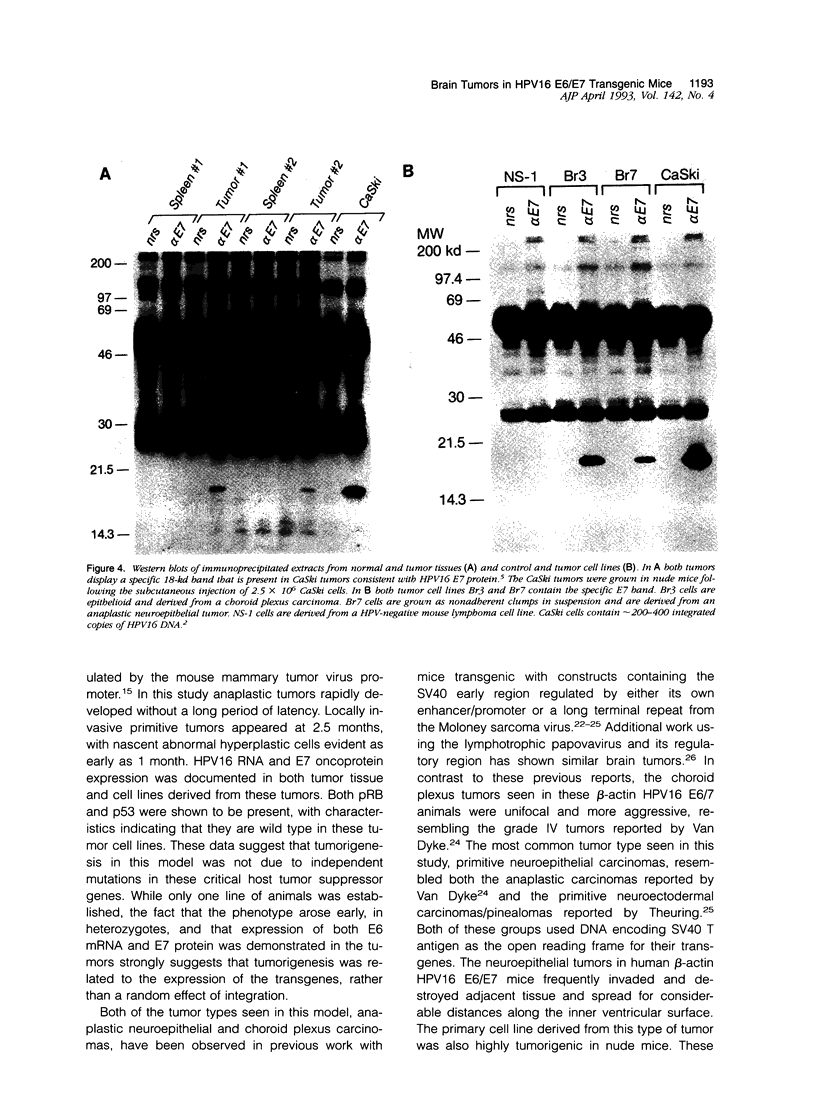
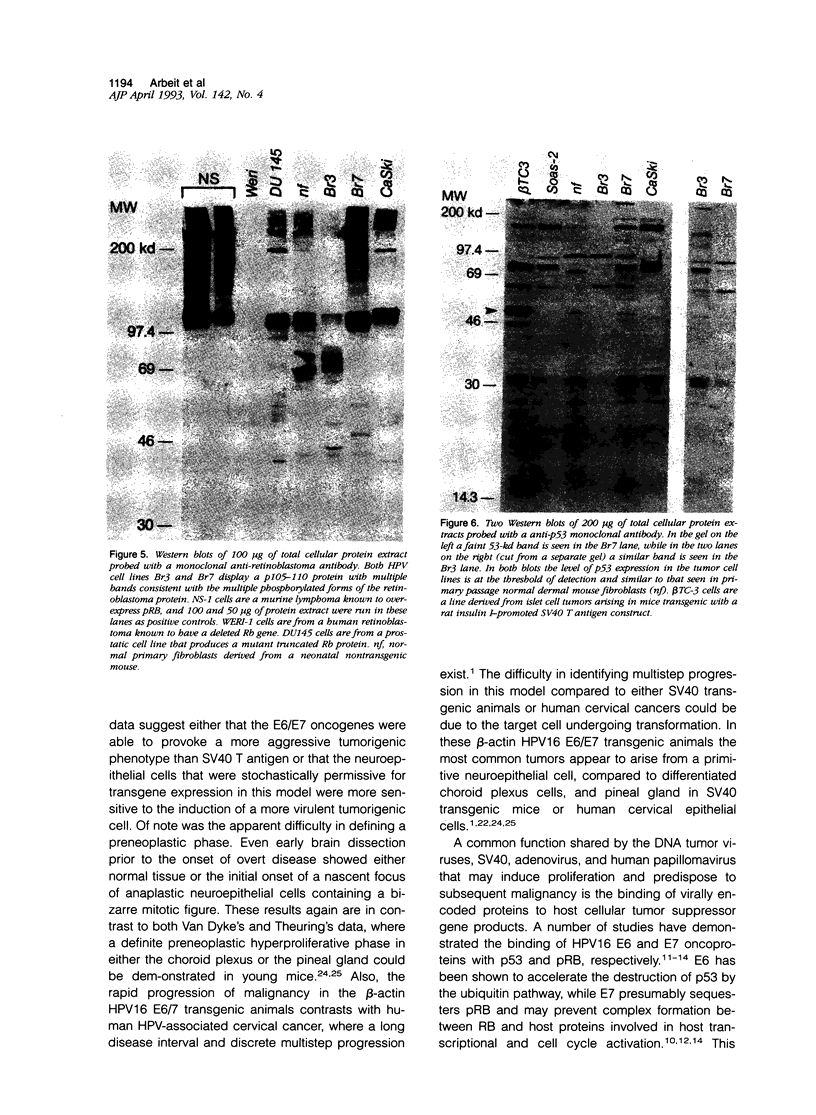



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Messing A., van Dyke T., Levine A. J., Palmiter R. D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984 Jun;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Van Dyke T. Uniform cell-autonomous tumorigenesis of the choroid plexus by papovavirus large T antigens. Mol Cell Biol. 1991 Dec;11(12):5968–5976. doi: 10.1128/mcb.11.12.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cripe T. P., Haugen T. H., Turk J. P., Tabatabai F., Schmid P. G., 3rd, Dürst M., Gissmann L., Roman A., Turek L. P. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987 Dec 1;6(12):3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P., Vousden K. H., Hubbert N. L., Lowy D. R., Schiller J. T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Bedell M. A., McCance D. J., Laiminis L. A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990 Feb;64(2):519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Dumanski J. P., Hansen M., Nordenskjold M., Collins V. P., Cavenee W. K. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988 Oct 1;48(19):5546–5551. [PubMed] [Google Scholar]
- Kondoh G., Murata Y., Aozasa K., Yutsudo M., Hakura A. Very high incidence of germ cell tumorigenesis (seminomagenesis) in human papillomavirus type 16 transgenic mice. J Virol. 1991 Jun;65(6):3335–3339. doi: 10.1128/jvi.65.6.3335-3339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner M. S., Mack D. H., Finicle A. B., Crook T., Vousden K. H., Laimins L. A. Human papillomavirus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription. EMBO J. 1992 Aug;11(8):3045–3052. doi: 10.1002/j.1460-2075.1992.tb05375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Matlashewski G., Schneider J., Banks L., Jones N., Murray A., Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 1987 Jun;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger A. K., Sheffield V. C., Duyk G., Daneshvar L., Edwards M. S., Cogen P. H. Identification of a germ-line mutation in the p53 gene in a patient with an intracranial ependymoma. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7825–7829. doi: 10.1073/pnas.88.17.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Teresky A. K., Levine A. J., Seiberg M. p53 mutations are not selected for in simian virus 40 T-antigen-induced tumors from transgenic mice. J Virol. 1992 Feb;66(2):641–649. doi: 10.1128/jvi.66.2.641-649.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Scheffner M., Huibregtse J. M., Howley P. M. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992;12:197–217. [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H., Eibl R. H., Wiestler O. D., Yasargil M. G., Newcomb E. W., Kleihues P. p53 mutations in nonastrocytic human brain tumors. Cancer Res. 1991 Nov 15;51(22):6202–6205. [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Messing A., Brinster R. L. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumours in transgenic mice. Nature. 1985 Aug 1;316(6027):457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- Raffel C., Gilles F. E., Weinberg K. I. Reduction to homozygosity and gene amplification in central nervous system primitive neuroectodermal tumors of childhood. Cancer Res. 1990 Feb 1;50(3):587–591. [PubMed] [Google Scholar]
- Rasheed B. K., Bigner S. H. Genetic alterations in glioma and medulloblastoma. Cancer Metastasis Rev. 1991 Dec;10(4):289–299. doi: 10.1007/BF00554791. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Prokoph H., Wettstein F. O. Oncogenic and nononcogenic human genital papillomaviruses generate the E7 mRNA by different mechanisms. J Virol. 1989 Mar;63(3):1441–1447. doi: 10.1128/jvi.63.3.1441-1447.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds H., Chen J. D., Van Dyke T. Complex formation between the lymphotropic papovavirus large tumor antigen and the tumor suppressor protein p53. J Virol. 1991 Oct;65(10):5417–5424. doi: 10.1128/jvi.65.10.5417-5424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuring F., Götz W., Balling R., Korf H. W., Schulze F., Herken R., Gruss P. Tumorigenesis and eye abnormalities in transgenic mice expressing MSV-SV40 large T-antigen. Oncogene. 1990 Feb;5(2):225–232. [PubMed] [Google Scholar]
- Thomas G. A., Raffel C. Loss of heterozygosity on 6q, 16q, and 17p in human central nervous system primitive neuroectodermal tumors. Cancer Res. 1991 Jan 15;51(2):639–643. [PubMed] [Google Scholar]
- Van Dyke T. A., Finlay C., Miller D., Marks J., Lozano G., Levine A. J. Relationship between simian virus 40 large tumor antigen expression and tumor formation in transgenic mice. J Virol. 1987 Jun;61(6):2029–2032. doi: 10.1128/jvi.61.6.2029-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991 May;5(5):714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]