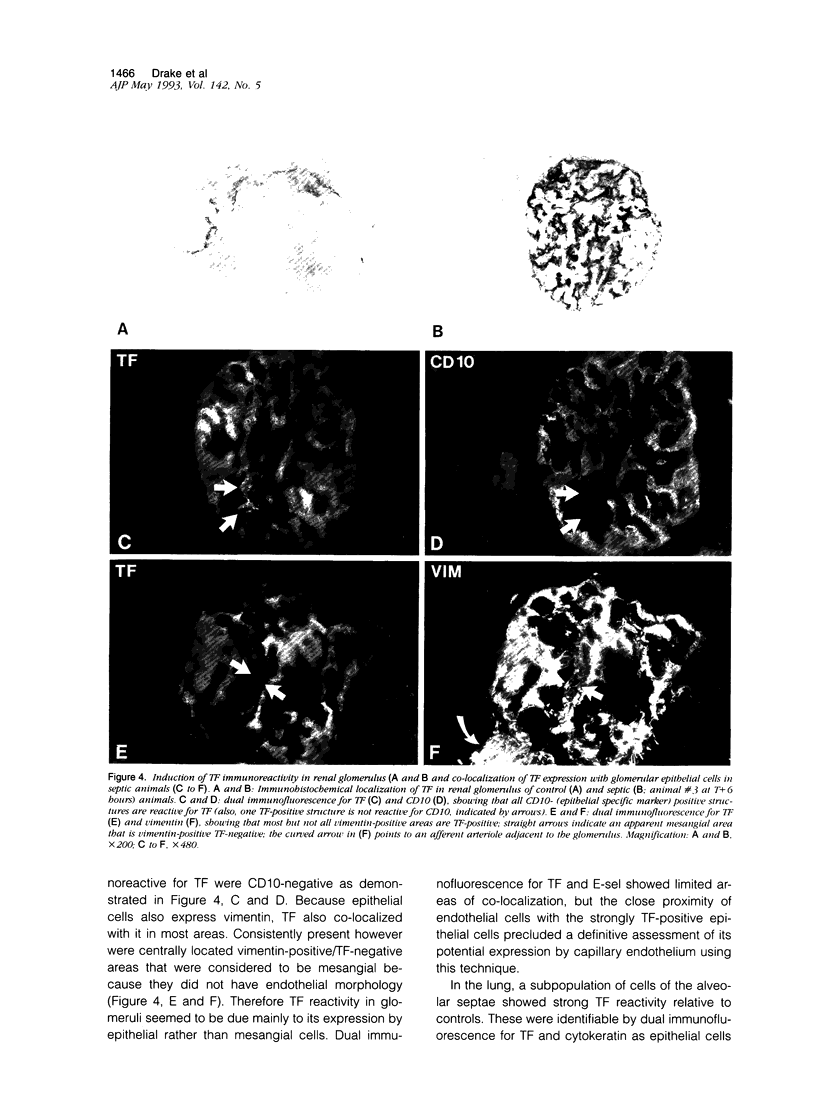
Abstract
Disseminated intravascular thrombosis is a frequent complication of endotoxic shock, and modulation of endothelial cell hemostatic properties has been proposed to play a role in its pathogenesis based on studies of endothelial cells in culture. This study examined the in vivo expression of tissue factor (TF) and thrombomodulin (TM) in a baboon model of lethal Escherichia coli sepsis using immunohistochemistry with monospecific antibodies. Expression of E-selectin (E-sel) was also determined as a marker of endothelial cell activation. Correlation of immunoreactivity with procoagulant activity in lipopolysaccharide-stimulated cultured human endothelial cells showed that immunohistochemistry was sufficiently sensitive to detect as little as 5% of the maximum in vitro endothelial cell TF response. Vascular endothelium of control animals expressed TM but had no detectable TF or E-sel. Following E. coli infusion, widespread E-sel expression and microvascular fibrin deposition was evident within 6 hours. However, expression of TF by endothelial cells became detectable only in the splenic microvasculature, where endothelial specificity of TF expression was confirmed by dual immunofluorescence of TF with von Willebrand's factor and with TM. In the spleen, there was a dissociation of expression of TF and E-sel, with marginal zone vessels being TF-positive and E-sel-negative, whereas sinusoidal endothelium was E-sel-positive but TF-negative. TM expression was unchanged from controls. Additionally, expression of TF by lung alveolar epithelial cells, splenic macrophages, and epithelial cells of the renal glomeruli was observed to be enhanced in septic animals. This study documents endothelial cell expression of TF in vivo in a relevant pathological setting. At the same time, compared with endothelial cells in culture, there is in vivo both significantly greater control of TF expression than expected, given the strong positive stimuli present in lethal E. coli septic shock and an unpredicted heterogeneity of activation responses.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985 Dec;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- Creasey A. A., Stevens P., Kenney J., Allison A. C., Warren K., Catlett R., Hinshaw L., Taylor F. B., Jr Endotoxin and cytokine profile in plasma of baboons challenged with lethal and sublethal Escherichia coli. Circ Shock. 1991 Feb;33(2):84–91. [PubMed] [Google Scholar]
- DeBault L. E., Esmon N. L., Olson J. R., Esmon C. T. Distribution of the thrombomodulin antigen in the rabbit vasculature. Lab Invest. 1986 Feb;54(2):172–178. [PubMed] [Google Scholar]
- Dittman W. A., Majerus P. W. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990 Jan 15;75(2):329–336. [PubMed] [Google Scholar]
- Drake T. A., Morrissey J. H., Edgington T. S. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989 May;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial and stromal cells. Infect Immun. 1989 Feb;57(2):507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Johnson A. E., Esmon N. L. Initiation of the protein C pathway. Ann N Y Acad Sci. 1991;614:30–43. doi: 10.1111/j.1749-6632.1991.tb43689.x. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Mason D. Y., Pulford K., Falini B., Bliss E., Gatter K. C., Stein H., Clarke L. C., McGee J. O. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986 Mar;54(3):322–335. [PubMed] [Google Scholar]
- Gross T. J., Simon R. H., Sitrin R. G. Tissue factor procoagulant expression by rat alveolar epithelial cells. Am J Respir Cell Mol Biol. 1992 Apr;6(4):397–403. doi: 10.1165/ajrcmb/6.4.397. [DOI] [PubMed] [Google Scholar]
- Kapiotis S., Besemer J., Bevec D., Valent P., Bettelheim P., Lechner K., Speiser W. Interleukin-4 counteracts pyrogen-induced downregulation of thrombomodulin in cultured human vascular endothelial cells. Blood. 1991 Jul 15;78(2):410–415. [PubMed] [Google Scholar]
- Mackman N., Brand K., Edgington T. S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991 Dec 1;174(6):1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee M. P., Wallin R., Wheeler F. B., Rothberger H. Initiation of the extrinsic pathway of coagulation by human and rabbit alveolar macrophages: a kinetic study. Blood. 1989 Oct;74(5):1583–1590. [PubMed] [Google Scholar]
- Moore K. L., Andreoli S. P., Esmon N. L., Esmon C. T., Bang N. U. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987 Jan;79(1):124–130. doi: 10.1172/JCI112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. L., Esmon C. T., Esmon N. L. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989 Jan;73(1):159–165. [PubMed] [Google Scholar]
- Morrissey J. H., Fair D. S., Edgington T. S. Monoclonal antibody analysis of purified and cell-associated tissue factor. Thromb Res. 1988 Nov 1;52(3):247–261. doi: 10.1016/0049-3848(88)90084-9. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerson Y. Tissue factor and hemostasis. Blood. 1988 Jan;71(1):1–8. [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Stages of renal ontogenesis identified by monoclonal antibodies reactive with lymphohemopoietic differentiation antigens. J Exp Med. 1983 Jan 1;157(1):155–172. doi: 10.1084/jem.157.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Rapaport S. I. The initiation of the tissue factor dependent pathway of blood coagulation. Adv Exp Med Biol. 1990;281:97–103. doi: 10.1007/978-1-4615-3806-6_10. [DOI] [PubMed] [Google Scholar]
- Redl H., Dinges H. P., Buurman W. A., van der Linden C. J., Pober J. S., Cotran R. S., Schlag G. Expression of endothelial leukocyte adhesion molecule-1 in septic but not traumatic/hypovolemic shock in the baboon. Am J Pathol. 1991 Aug;139(2):461–466. [PMC free article] [PubMed] [Google Scholar]
- Rodgers G. M. Hemostatic properties of normal and perturbed vascular cells. FASEB J. 1988 Feb;2(2):116–123. doi: 10.1096/fasebj.2.2.3277885. [DOI] [PubMed] [Google Scholar]
- Schmidt E. E., MacDonald I. C., Groom A. C. Microcirculatory pathways in normal human spleen, demonstrated by scanning electron microscopy of corrosion casts. Am J Anat. 1988 Mar;181(3):253–266. doi: 10.1002/aja.1001810304. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Skalli O., Gabbiani G. Distribution of intermediate filament proteins in normal and diseased human glomeruli. Am J Pathol. 1986 Dec;125(3):465–475. [PMC free article] [PubMed] [Google Scholar]
- Steinberg S. F., Robinson R. B., Lieberman H. B., Stern D. M., Rosen M. R. Thrombin modulates phosphoinositide metabolism, cytosolic calcium, and impulse initiation in the heart. Circ Res. 1991 May;68(5):1216–1229. doi: 10.1161/01.res.68.5.1216. [DOI] [PubMed] [Google Scholar]
- Taylor F. B., Jr, Chang A., Ruf W., Morrissey J. H., Hinshaw L., Catlett R., Blick K., Edgington T. S. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991 Mar;33(3):127–134. [PubMed] [Google Scholar]
- Voss B. L., De Bault L. E., Blick K. E., Chang A. C., Stiers D. L., Hinshaw L. B., Taylor F. B. Sequential renal alterations in septic shock in the primate. Circ Shock. 1991 Mar;33(3):142–155. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]