Abstract
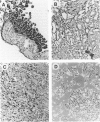
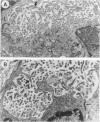
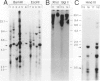
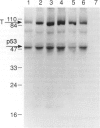
In the course of studies to elucidate the relative contribution of simian virus 40 (SV40) large T and small t proteins during oncogenesis, we observed the appearance of pericardial and pleural tumors in 100% of Syrian hamsters injected in the pleural space with wild type SV40. When SV40 was injected via the intracardiac or intraperitoneal routes, more than 50% of hamsters developed mesothelial tumors. Macroscopic, microscopic, ultramicroscopic, and histochemical characteristics identify these neoplasms and derived cell lines as mesotheliomas and mesothelioma-derived cell lines. The SV40 genome was integrated and expressed in the mesotheliomas and derived cell lines. The absence of mesotheliomas in hamsters injected with SV40 small t deletion mutants indicates that the small t protein plays an important role in the development of SV40-induced mesotheliomas. To the best of our knowledge, this is the first definitive report of virus-induced mesotheliomas in mammals.
Full text
PDF



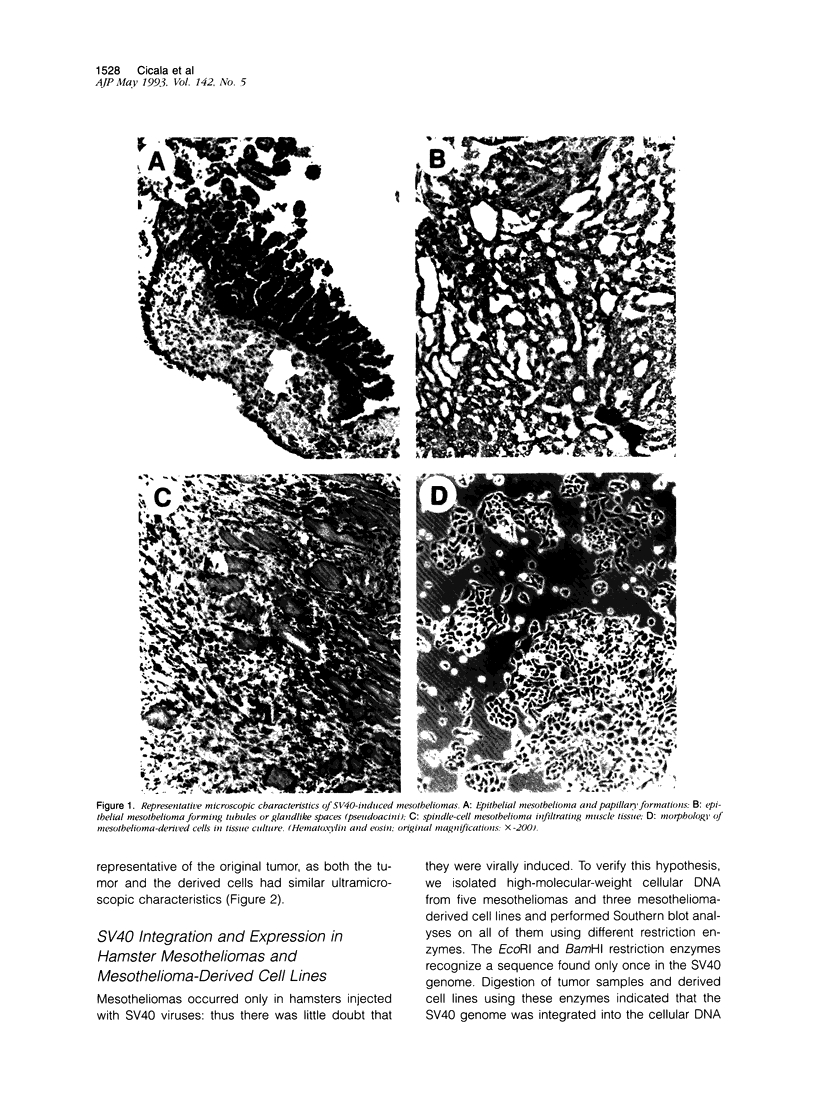
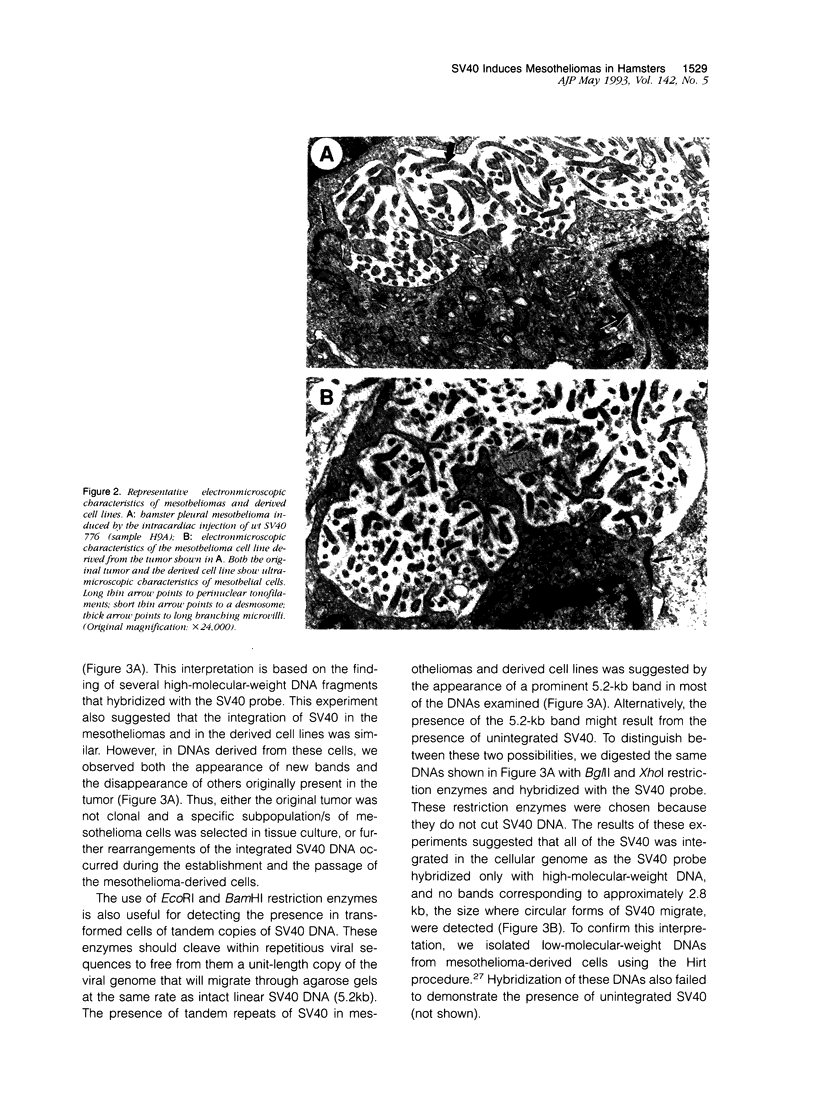
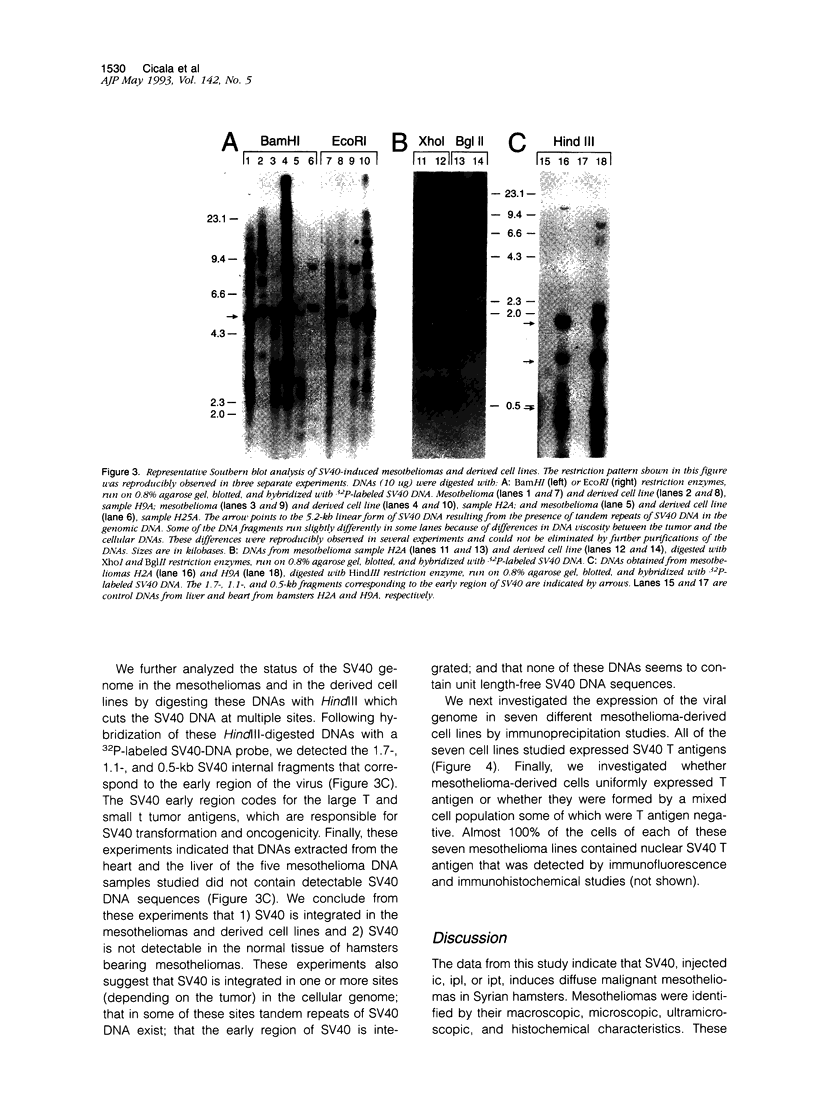
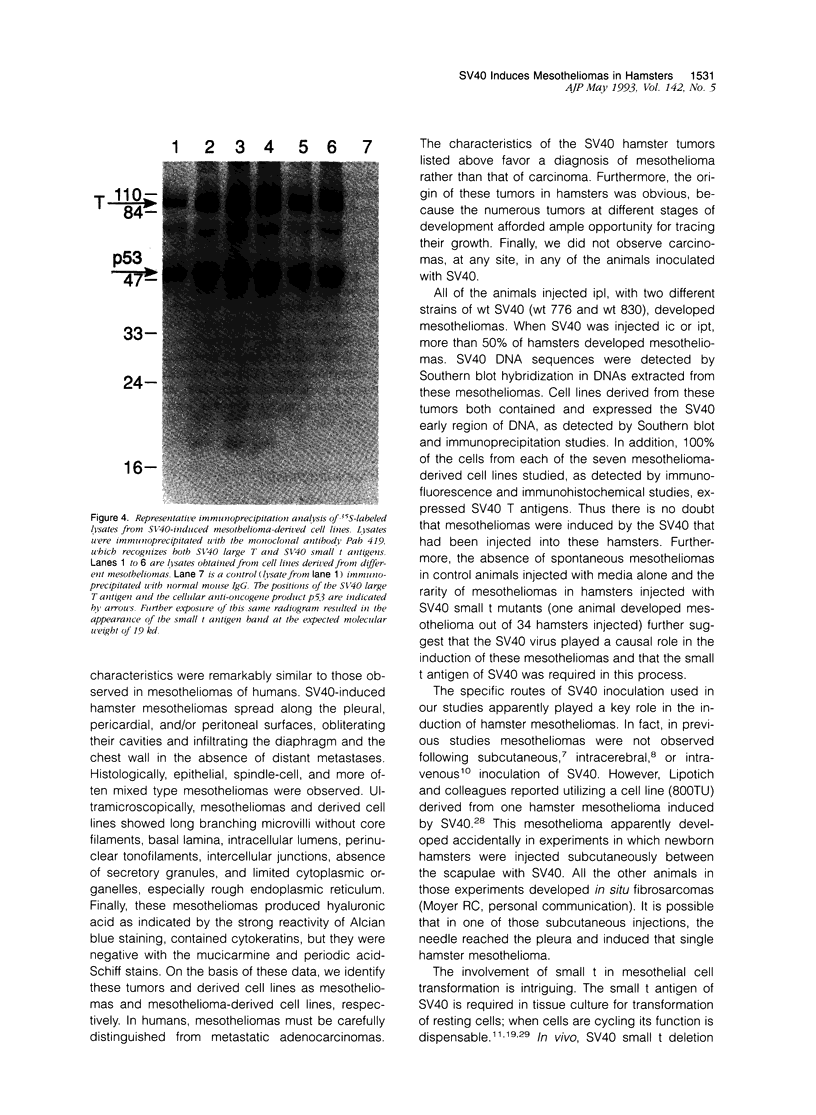


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Chesterman F. C., Baron S. Induction of tumors in adult hamsters with simian virus 40. J Natl Cancer Inst. 1967 Apr;38(4):567–572. [PubMed] [Google Scholar]
- Bergsagel D. J., Finegold M. J., Butel J. S., Kupsky W. J., Garcea R. L. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992 Apr 9;326(15):988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- Carbone M., Hauser J., Carty M. P., Rundell K., Dixon K., Levine A. S. Simian virus 40 (SV40) small t antigen inhibits SV40 DNA replication in vitro. J Virol. 1992 Mar;66(3):1804–1808. doi: 10.1128/jvi.66.3.1804-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M., Lewis A. M., Jr, Matthews B. J., Levine A. S., Dixon K. Characterization of hamster tumors induced by simian virus 40 small t deletion mutants as true histiocytic lymphomas. Cancer Res. 1989 Mar 15;49(6):1565–1571. [PubMed] [Google Scholar]
- Chabot J. F., Beard D., Langlois A. J., Beard J. W. Mesotheliomas of peritoneum, epicardium, and pericardium induced by strain MC29 avian leukosis virus. Cancer Res. 1970 May;30(5):1287–1308. [PubMed] [Google Scholar]
- Cicala C., Pompetti F., Nguyen P., Dixon K., Levine A. S., Carbone M. SV40 small t deletion mutants preferentially transform mononuclear phagocytes and B lymphocytes in vivo. Virology. 1992 Sep;190(1):475–479. doi: 10.1016/0042-6822(92)91237-o. [DOI] [PubMed] [Google Scholar]
- Dardick I., Jabi M., McCaughey W. T., Deodhare S., van Nostrand A. W., Srigley J. R. Diffuse epithelial mesothelioma: a review of the ultrastructural spectrum. Ultrastruct Pathol. 1987;11(5-6):503–533. doi: 10.3109/01913128709048446. [DOI] [PubMed] [Google Scholar]
- Diamandopoulos G. T. Leukemia, lymphoma, and osteosarcoma induced in the Syrian golden hamster by simian virus 40. Science. 1972 Apr 14;176(4031):173–175. doi: 10.1126/science.176.4031.173. [DOI] [PubMed] [Google Scholar]
- EDDY B. E. SIMIAN VIRUS 40 (SV-40): AN ONCOGENIC VIRUS. Prog Exp Tumor Res. 1964;4:1–26. doi: 10.1159/000385971. [DOI] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992 Mar;66(3):1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER P., KIRSCHSTEIN R. L. SV40-induced ependymomas in newborn hamsters. I. Virus-tumor relationships. Virology. 1962 Dec;18:582–588. doi: 10.1016/0042-6822(62)90061-2. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Dependence of asbestos- and mineral dust-induced transformation of mammalian cells in culture on fiber dimension. Cancer Res. 1984 May;44(5):2170–2180. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jaurand M. C., Fleury J., Monchaux G., Nebut M., Bignon J. Pleural carcinogenic potency of mineral fibers (asbestos, attapulgite) and their cytotoxicity on cultured cells. J Natl Cancer Inst. 1987 Oct;79(4):797–804. [PubMed] [Google Scholar]
- Ke Y., Reddel R. R., Gerwin B. I., Reddel H. K., Somers A. N., McMenamin M. G., LaVeck M. A., Stahel R. A., Lechner J. F., Harris C. C. Establishment of a human in vitro mesothelial cell model system for investigating mechanisms of asbestos-induced mesothelioma. Am J Pathol. 1989 May;134(5):979–991. [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Martin R. G. Oncogenicity of simian virus 40 deletion mutants that induce altered 17-kilodalton t-proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4299–4302. doi: 10.1073/pnas.76.9.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipotich G., Moyer M. P., Moyer R. C. Rescue of SV40 following transfection of TC7 cells with cellular DNAs containing complete and partial SV40 genomes. Mol Gen Genet. 1982;186(1):78–81. doi: 10.1007/BF00422915. [DOI] [PubMed] [Google Scholar]
- Matthews B. J., Levine A. S., Dixon K. Deletion mutations in the small t antigen gene alter the tissue specificity of tumors induced by simian virus 40. J Virol. 1987 Apr;61(4):1282–1285. doi: 10.1128/jvi.61.4.1282-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey W. T., Colby T. V., Battifora H., Churg A., Corson J. M., Greenberg S. D., Grimes M. M., Hammar S., Roggli V. L., Unni K. K. Diagnosis of diffuse malignant mesothelioma: experience of a US/Canadian Mesothelioma Panel. Mod Pathol. 1991 May;4(3):342–353. [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Peterson J. T., Jr, Greenberg S. D., Buffler P. A. Non-asbestos-related malignant mesothelioma. A review. Cancer. 1984 Sep 1;54(5):951–960. doi: 10.1002/1097-0142(19840901)54:5<951::aid-cncr2820540536>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Malan-Shibley L., Gerwin B. I., Metcalf R. A., Harris C. C. Tumorigenicity of human mesothelial cell line transfected with EJ-ras oncogene. J Natl Cancer Inst. 1989 Jun 21;81(12):945–948. doi: 10.1093/jnci/81.12.945. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. H., Deutsch J. F., Ungers G. E. Growth and purification of SV40 virus for biochemical studies. J Virol Methods. 1981 Oct;3(3):167–176. doi: 10.1016/0166-0934(81)90051-3. [DOI] [PubMed] [Google Scholar]
- STANTON M. F., STEWART S. E., EDDY B. E., BLACKWELL R. H. Oncogenic effect of tissue-culture preparations of polyoma virus on fetal mice. J Natl Cancer Inst. 1959 Dec;23:1441–1475. [PubMed] [Google Scholar]
- Scheidtmann K. H., Mumby M. C., Rundell K., Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Simian virus 40 small t antigen is not required for the maintenance of transformation but may act as a promoter (cocarcinogen) during establishment of transformation in resting rat cells. J Virol. 1979 Dec;32(3):979–988. doi: 10.1128/jvi.32.3.979-988.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Kannerstein M. Ultrastructure of human malignant diffuse mesothelioma. Am J Pathol. 1976 Nov;85(2):241–262. [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Shenk T. Nucleotide sequence analysis of viable deletion mutants lacking segments of the simian virus 40 genome coding for small t antigen. J Virol. 1979 Jun;30(3):668–673. doi: 10.1128/jvi.30.3.668-673.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Ruediger R., Slaughter C., Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann L. B., Antman K. H. Incidence, presentation and promising new treatments for malignant mesothelioma. Oncology (Williston Park) 1989 Jan;3(1):67-72; discussion 73-4, 77. [PubMed] [Google Scholar]
- Yang S. I., Lickteig R. L., Estes R., Rundell K., Walter G., Mumby M. C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]