Abstract
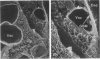
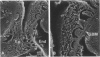
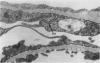
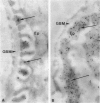
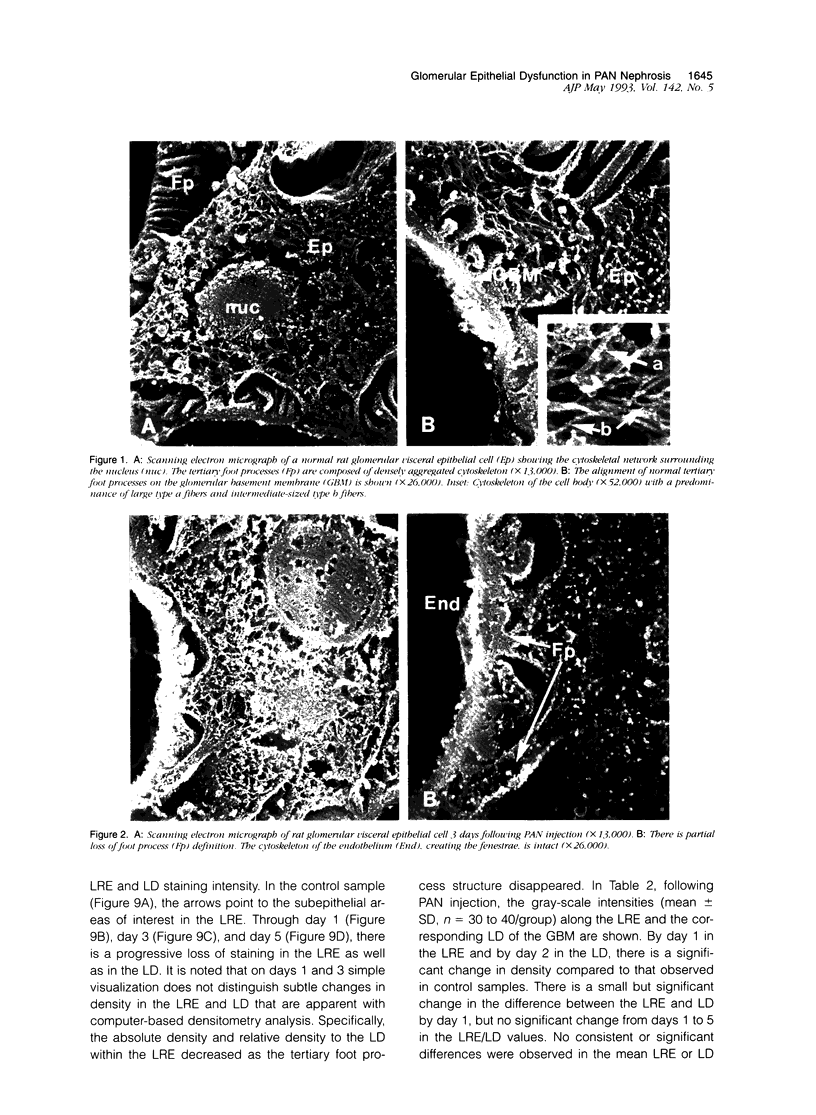
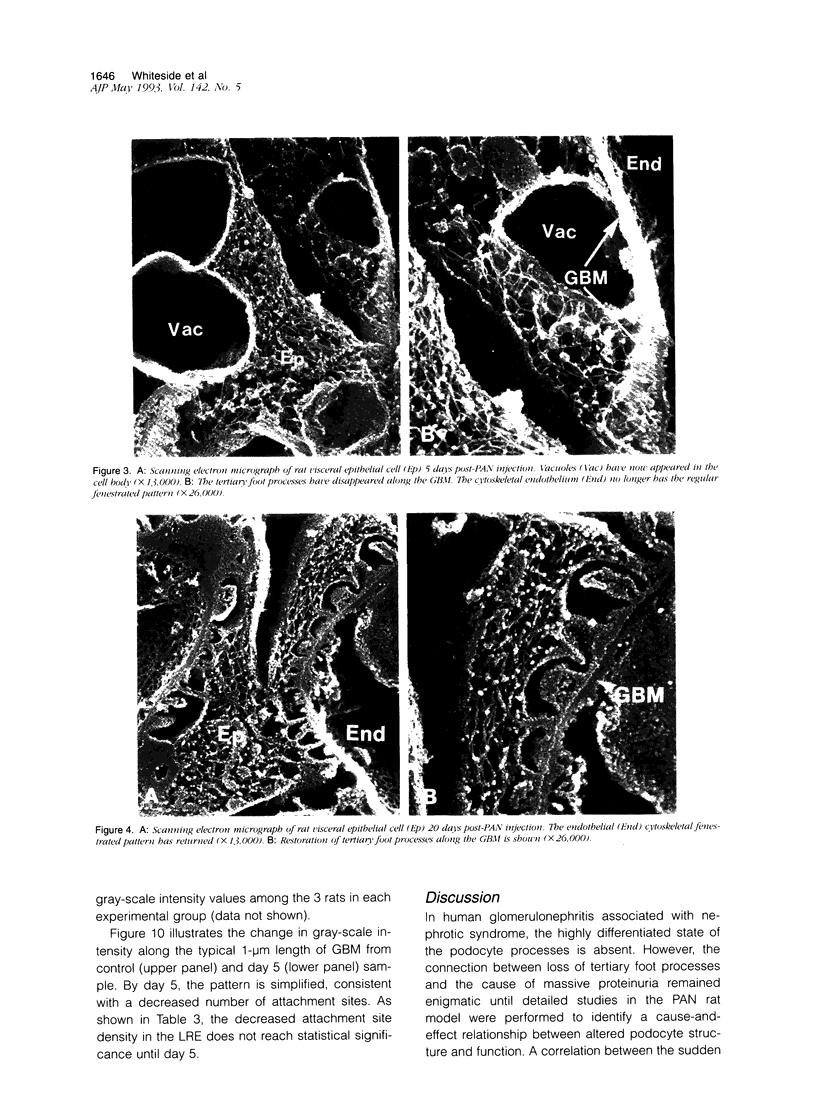
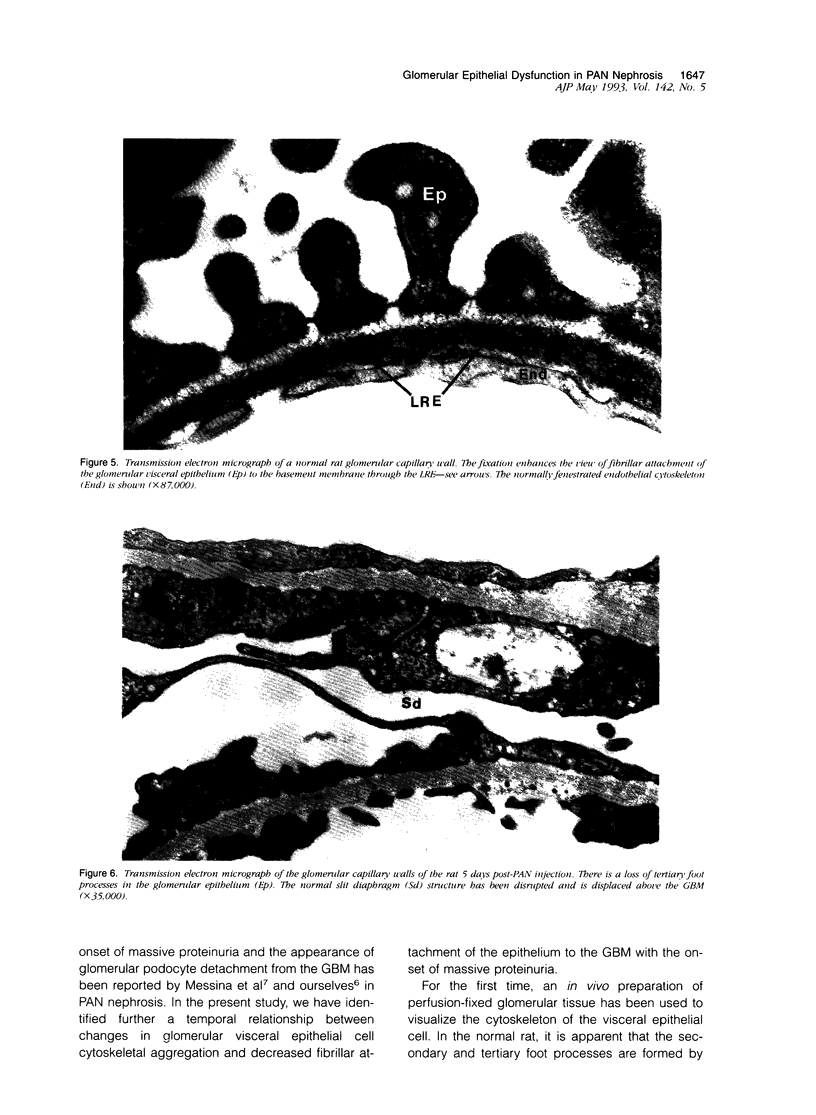
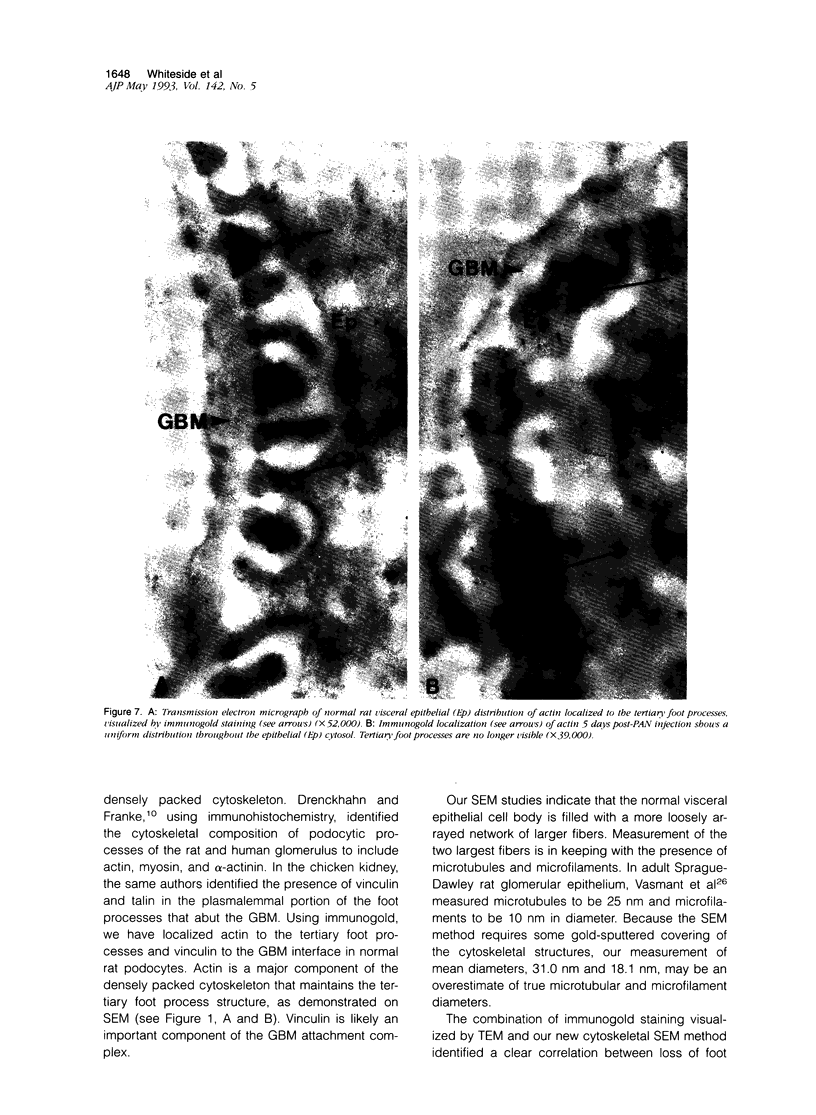
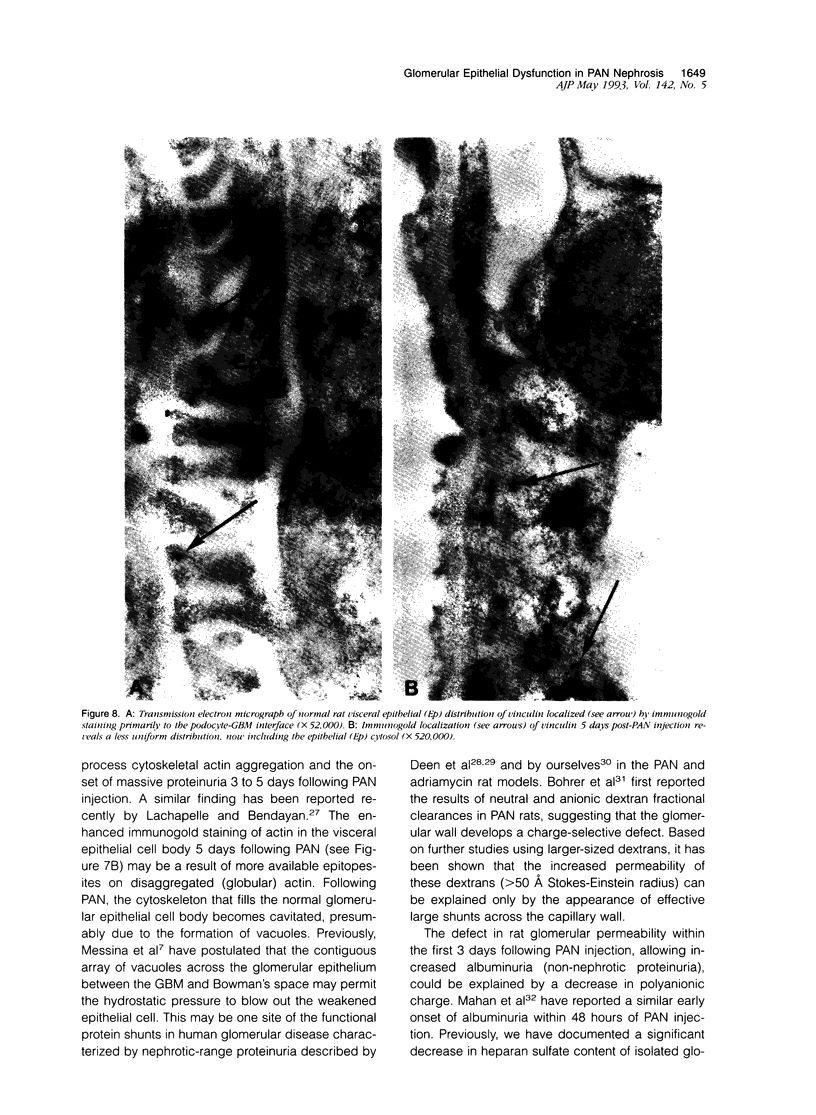
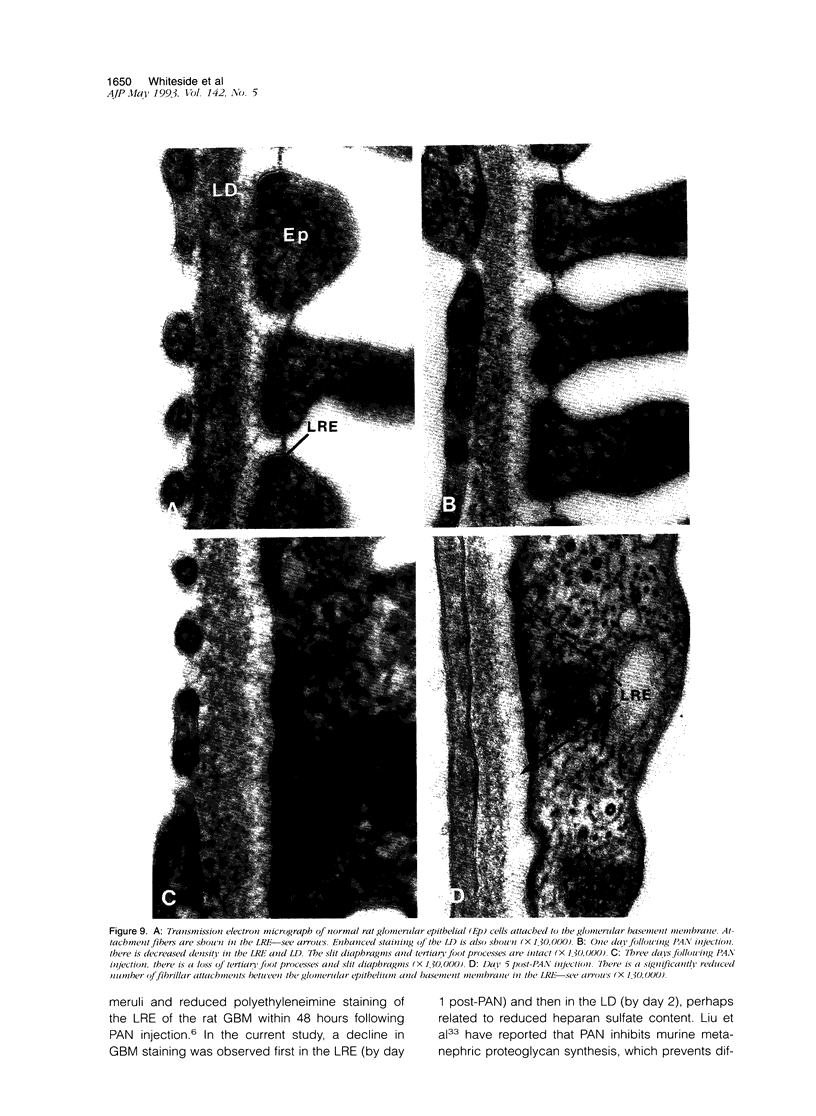
Puromycin aminonucleoside--(PAN) treated rats develop acute nephrotic syndrome, mimicking human minimal lesion disease. In PAN nephrosis, podocyte detachment from the glomerular basement membrane (GBM) is the most likely cause of massive proteinuria in this model. To elucidate further the mechanisms of PAN-induced cellular dysfunction, new methods were employed to visualize podocyte cytoskeletal aggregation and to measure fibrillar attachment to the GBM. Adult Sprague-Dawley rats (n = 4/group) received a single tail-vein injection of PAN (75 mg/kg). On days 1, 2, 3, and 5 following injection, 24-hour urine collections were obtained for creatinine clearance, albuminuria, and total proteinuria. Then kidneys from each group were fixed by perfusion. Podocytic cytoskeleton was visualized by scanning electron microscopy. Subepithelial GBM staining and attachment fiber number, observed on digitized images of transmission electron micrographs, were quantitated with computer-based density analysis. A significant reduction in attachment fiber number in the GBM lamina rara externa occurred by day 5. On scanning electron micrographs, the secondary and tertiary podocytic processes were observed to be formed by highly aggregated cytoskeleton, which became partially disaggregated by day 3, was totally absent by day 5, and normalized by day 20. Immunogold staining revealed that actin and vinculin localized to the tertiary podocytic processes in the normal state were dispersed into the cell body following PAN. Podocyte cytoskeletal disaggregation precedes, and detachment from the GBM occurs simultaneously with, the onset of massive proteinuria in the PAN model.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avasthi P. S., Evan A. P. Glomerular permeability in aminonucleoside-induced nephrosis in rats. A proposed role of endothelial cells. J Lab Clin Med. 1979 Feb;93(2):266–276. [PubMed] [Google Scholar]
- Bacallao R., Fine L. G. Molecular events in the organization of renal tubular epithelium: from nephrogenesis to regeneration. Am J Physiol. 1989 Dec;257(6 Pt 2):F913–F924. doi: 10.1152/ajprenal.1989.257.6.F913. [DOI] [PubMed] [Google Scholar]
- Bell P. B., Jr, Lindroth M., Fredriksson B. A., Liu X. D. Problems associated with the preparation of whole mounts of cytoskeletons for high resolution electron microscopy. Scanning Microsc Suppl. 1989;3:117–135. [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Robertson C. R., Brenner B. M., Troy J. L., Willis W. T. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J Clin Invest. 1977 Jul;60(1):152–161. doi: 10.1172/JCI108751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Bridges C. R., Brenner B. M., Myers B. D. Heteroporous model of glomerular size selectivity: application to normal and nephrotic humans. Am J Physiol. 1985 Sep;249(3 Pt 2):F374–F389. doi: 10.1152/ajprenal.1985.249.3.F374. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Franke R. P. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988 Nov;59(5):673–682. [PubMed] [Google Scholar]
- Geoghegan W. D., Ackerman G. A. Adsorption of horseradish peroxidase, ovomucoid and anti-immunoglobulin to colloidal gold for the indirect detection of concanavalin A, wheat germ agglutinin and goat anti-human immunoglobulin G on cell surfaces at the electron microscopic level: a new method, theory and application. J Histochem Cytochem. 1977 Nov;25(11):1187–1200. doi: 10.1177/25.11.21217. [DOI] [PubMed] [Google Scholar]
- Grishman E., Churg J. Focal glomerular sclerosis in nephrotic patients: an electron microscopic study of glomerular podocytes. Kidney Int. 1975 Feb;7(2):111–122. doi: 10.1038/ki.1975.16. [DOI] [PubMed] [Google Scholar]
- Groggel G. C., Hovingh P., Border W. A., Linker A. Changes in glomerular heparan sulfate in puromycin aminonucleoside nephrosis. Am J Pathol. 1987 Sep;128(3):521–527. [PMC free article] [PubMed] [Google Scholar]
- Kasinath B. S., Singh A. K., Kanwar Y. S., Lewis E. J. Effect of puromycin aminonucleoside on HSPG core protein content of glomerular epithelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):F590–F596. doi: 10.1152/ajprenal.1988.255.4.F590. [DOI] [PubMed] [Google Scholar]
- Lachapelle M., Bendayan M. Contractile proteins in podocytes: immunocytochemical localization of actin and alpha-actinin in normal and nephrotic rat kidneys. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(2):105–111. doi: 10.1007/BF02899534. [DOI] [PubMed] [Google Scholar]
- Liu Z. Z., Dalecki T. M., Kashihara N., Wallner E. I., Kanwar Y. S. Effect of puromycin on metanephric differentiation: morphological, autoradiographic and biochemical studies. Kidney Int. 1991 Jun;39(6):1140–1155. doi: 10.1038/ki.1991.145. [DOI] [PubMed] [Google Scholar]
- Mahan J. D., Sisson-Ross S., Vernier R. L. Glomerular basement membrane anionic charge site changes early in aminonucleoside nephrosis. Am J Pathol. 1986 Nov;125(2):393–401. [PMC free article] [PubMed] [Google Scholar]
- Mbassa G., Elger M., Kriz W. The ultrastructural organization of the basement membrane of Bowman's capsule in the rat renal corpuscle. Cell Tissue Res. 1988 Jul;253(1):151–163. doi: 10.1007/BF00221750. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Messina A., Davies D. J., Dillane P. C., Ryan G. B. Glomerular epithelial abnormalities associated with the onset of proteinuria in aminonucleoside nephrosis. Am J Pathol. 1987 Feb;126(2):220–229. [PMC free article] [PubMed] [Google Scholar]
- Molitoris B. A., Hoilien C. A., Dahl R., Ahnen D. J., Wilson P. D., Kim J. Characterization of ischemia-induced loss of epithelial polarity. J Membr Biol. 1988 Dec;106(3):233–242. doi: 10.1007/BF01872161. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Kinne R. Ischemia induces surface membrane dysfunction. Mechanism of altered Na+-dependent glucose transport. J Clin Invest. 1987 Sep;80(3):647–654. doi: 10.1172/JCI113117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Modulation of fodrin (membrane skeleton) stability by cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1987 Jun;104(6):1527–1537. doi: 10.1083/jcb.104.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Kawakami K., Miyao M., Oite T. Ultrastructural alterations of glomerular anionic sites in idiopathic membranous glomerulonephritis. Clin Nephrol. 1986 Jul;26(1):7–14. [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975 Oct;8(4):219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- Sakai T., Kriz W. The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl) 1987;176(3):373–386. doi: 10.1007/BF00310191. [DOI] [PubMed] [Google Scholar]
- Saunders S., Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol. 1988 Feb;106(2):423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., Sawada H., Farquhar M. G. Basement membrane heparan sulfate proteoglycans are concentrated in the laminae rarae and in podocytes of the rat renal glomerulus. Proc Natl Acad Sci U S A. 1985 May;82(10):3296–3300. doi: 10.1073/pnas.82.10.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlanovich S., Deen W. M., Jones H. W., 3rd, Schwartz H. C., Myers B. D. Functional nature of glomerular injury in progressive diabetic glomerulopathy. Diabetes. 1987 May;36(5):556–565. doi: 10.2337/diab.36.5.556. [DOI] [PubMed] [Google Scholar]
- Vasmant D., Maurice M., Feldmann G. Cytoskeleton ultrastructure of podocytes and glomerular endothelial cells in man and in the rat. Anat Rec. 1984 Sep;210(1):17–24. doi: 10.1002/ar.1092100104. [DOI] [PubMed] [Google Scholar]
- Venaille T. J., Mendis A. H., Warton A., Walker L., Papadimitriou J. M., Robinson B. W. Study of human epithelial cell detachment and damage: development of a model. Immunol Cell Biol. 1989 Dec;67(Pt 6):359–369. doi: 10.1038/icb.1989.52. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Cotran R. S., Karnovsky M. J. An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J Exp Med. 1970 Dec 1;132(6):1168–1180. doi: 10.1084/jem.132.6.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani R. R., Hawkins E. P. Recurrent focal segmental glomerulosclerosis. A pathological study of the early lesion. Am J Nephrol. 1986;6(4):263–270. doi: 10.1159/000167173. [DOI] [PubMed] [Google Scholar]
- Whiteside C. I., Thompson J. The role of angiotensin-II in progressive diabetic glomerulopathy in the rat. Endocrinology. 1989 Oct;125(4):1932–1940. doi: 10.1210/endo-125-4-1932. [DOI] [PubMed] [Google Scholar]
- Whiteside C., Prutis K., Cameron R., Thompson J. Glomerular epithelial detachment, not reduced charge density, correlates with proteinuria in adriamycin and puromycin nephrosis. Lab Invest. 1989 Dec;61(6):650–660. [PubMed] [Google Scholar]
- Yoshikawa N., Ito H., Akamatsu R., Hazikano H., Okada S., Matsuo T. Glomerular podocyte vacuolation in focal segmental glomerulosclerosis. Arch Pathol Lab Med. 1986 May;110(5):394–398. [PubMed] [Google Scholar]