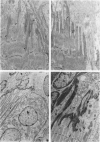
Abstract
The myotendinous junction (MTJ) is the major site of force transmission from myofibrils across the muscle cell membrane to the extracellular matrix. The MTJ is thus an appropriate model system in which to test the hypothesis that dystrophin, the gene product absent in Duchenne muscular dystrophy, functions as a structural link between the muscle cytoskeleton and the cell membrane. We studied changes in MTJ structure in dystrophin-deficient mdx mice during periods of growth and aging that spanned prenecrotic, necrotic, and regenerative phases of postnatal muscle development in mdx mice. Prenecrotic animals were found to exhibit structural defects at MTJs that were similar to those described previously in animals at the peak of necrosis, including a reduction in lateral associations between thin filaments and the MTJ membrane. These defects therefore occur before necrosis and may be directly related to the absence of dystrophin. Observations of regenerating and fully regenerated MTJs in adult animals show that the defects are still present, indicating that normal thin filament-membrane associations are never formed in dystrophin-deficient muscle. However, in prenecrotic as well as regenerated adult mdx muscle, the MTJ membrane is only slightly less folded than in age-matched controls. This indicates that mdx muscle possesses some dystrophin-independent mechanism that allows for the initial formation of MTJs, despite the absence of dystrophin. The presence of the defect in normal, lateral, thin filament-membrane associations in mdx muscle, regardless of age, supports the hypothesis that dystrophin functions as a structural link between thin filaments and the membrane.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLBROOK D. An electron microscopic study of regenerating skeletal muscle. J Anat. 1962 Apr;96:137–152. [PMC free article] [PubMed] [Google Scholar]
- Almekinders L. C., Gilbert J. A. Healing of experimental muscle strains and the effects of nonsteroidal antiinflammatory medication. Am J Sports Med. 1986 Jul-Aug;14(4):303–308. doi: 10.1177/036354658601400411. [DOI] [PubMed] [Google Scholar]
- Anderson J. E., Bressler B. H., Ovalle W. K. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil. 1988 Dec;9(6):499–515. doi: 10.1007/BF01738755. [DOI] [PubMed] [Google Scholar]
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Beckerle M. C., Burridge K., DeMartino G. N., Croall D. E. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987 Nov 20;51(4):569–577. doi: 10.1016/0092-8674(87)90126-7. [DOI] [PubMed] [Google Scholar]
- Bodensteiner J. B., Engel A. G. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978 May;28(5):439–446. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- Burridge K., Mangeat P. An interaction between vinculin and talin. Nature. 1984 Apr 19;308(5961):744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Kunkel L. M., Watkins S. C. The subcellular distribution of dystrophin in mouse skeletal, cardiac, and smooth muscle. J Cell Biol. 1991 Oct;115(2):411–421. doi: 10.1083/jcb.115.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Carpenter S., Karpati G. Duchenne muscular dystrophy: plasma membrane loss initiates muscle cell necrosis unless it is repaired. Brain. 1979 Mar;102(1):147–161. doi: 10.1093/brain/102.1.147. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. doi: 10.1016/s0065-2776(08)60443-5. [DOI] [PubMed] [Google Scholar]
- Coulton G. R., Curtin N. A., Morgan J. E., Partridge T. A. The mdx mouse skeletal muscle myopathy: II. Contractile properties. Neuropathol Appl Neurobiol. 1988 Jul-Aug;14(4):299–314. doi: 10.1111/j.1365-2990.1988.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Fulthorpe J. J. Stages in fibre breakdown in Duchenne muscular dystrophy. An electron-microscopic study. J Neurol Sci. 1975 Feb;24(2):179–200. doi: 10.1016/0022-510x(75)90232-4. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Jaros E. Ultrastructure of the skeletal muscle in the X chromosome-linked dystrophic (mdx) mouse. Comparison with Duchenne muscular dystrophy. Acta Neuropathol. 1988;77(1):69–81. doi: 10.1007/BF00688245. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Walsh J., Nicholson L. V., Harris J. B. Ultrastructural localization of dystrophin in human muscle by using gold immunolabelling. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):197–210. doi: 10.1098/rspb.1990.0034. [DOI] [PubMed] [Google Scholar]
- Daems W. T. On the fine structure of human neutrophilic leukocyte granules. J Ultrastruct Res. 1968 Aug;24(3):343–348. doi: 10.1016/s0022-5320(68)90070-1. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Evans R. R., Robson R. M., Stromer M. H. Properties of smooth muscle vinculin. J Biol Chem. 1984 Mar 25;259(6):3916–3924. [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hudson G. Quantitative study of the eosinophil granulocytes. Semin Hematol. 1968 Apr;5(2):166–186. [PubMed] [Google Scholar]
- Hurme T., Kalimo H. Adhesion in skeletal muscle during regeneration. Muscle Nerve. 1992 Apr;15(4):482–489. doi: 10.1002/mus.880150412. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992 Feb 20;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Khurana T. S., Watkins S. C., Chafey P., Chelly J., Tomé F. M., Fardeau M., Kaplan J. C., Kunkel L. M. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul Disord. 1991;1(3):185–194. doi: 10.1016/0960-8966(91)90023-l. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Mackay B., Harrop T. J., Muir A. R. The fine structure of the muscle tendon junction in the rat. Acta Anat (Basel) 1969;73(4):588–602. doi: 10.1159/000143318. [DOI] [PubMed] [Google Scholar]
- Mair W. G., Tomé F. M. The ultrastructure of the adult and developing human myotendinous junction. Acta Neuropathol. 1972;21(3):239–252. doi: 10.1007/BF00688503. [DOI] [PubMed] [Google Scholar]
- Mastaglia F. L., Papadimitriou J. M., Kakulas B. A. Regeneration of muscle in Duchenne muscular dystrophy: an electron microscope study. J Neurol Sci. 1970 Nov;11(5):425–444. doi: 10.1016/0022-510x(70)90002-x. [DOI] [PubMed] [Google Scholar]
- Nguyen T. M., Ellis J. M., Love D. R., Davies K. E., Gatter K. C., Dickson G., Morris G. E. Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol. 1991 Dec;115(6):1695–1700. doi: 10.1083/jcb.115.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Campbell K. P. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol. 1991 Dec;115(6):1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Matsumura K., Kahl S. D., Leveille C. J., Campbell K. P. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 1991 Sep;7(3):499–508. doi: 10.1016/0896-6273(91)90301-f. [DOI] [PubMed] [Google Scholar]
- Ovalle W. K. The human muscle-tendon junction. A morphological study during normal growth and at maturity. Anat Embryol (Berl) 1987;176(3):281–294. doi: 10.1007/BF00310184. [DOI] [PubMed] [Google Scholar]
- Pons F., Augier N., Léger J. O., Robert A., Tomé F. M., Fardeau M., Voit T., Nicholson L. V., Mornet D., Léger J. J. A homologue of dystrophin is expressed at the neuromuscular junctions of normal individuals and DMD patients, and of normal and mdx mice. Immunological evidence. FEBS Lett. 1991 Apr 22;282(1):161–165. doi: 10.1016/0014-5793(91)80468-i. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Kasper C. E., Greaser M. L., Moss R. L. Functional significance of myosin transitions in single fibers of developing soleus muscle. Am J Physiol. 1988 May;254(5 Pt 1):C605–C613. doi: 10.1152/ajpcell.1988.254.5.C605. [DOI] [PubMed] [Google Scholar]
- Samitt C. E., Bonilla E. Immunocytochemical study of dystrophin at the myotendinous junction. Muscle Nerve. 1990 Jun;13(6):493–500. doi: 10.1002/mus.880130605. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. The morphology of regeneration of skeletal muscles in the rat. Tissue Cell. 1976;8(4):673–692. doi: 10.1016/0040-8166(76)90039-2. [DOI] [PubMed] [Google Scholar]
- Shear C. R., Bloch R. J. Vinculin in subsarcolemmal densities in chicken skeletal muscle: localization and relationship to intracellular and extracellular structures. J Cell Biol. 1985 Jul;101(1):240–256. doi: 10.1083/jcb.101.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J. G., Law D. J. Dystrophin is required for normal thin filament-membrane associations at myotendinous junctions. Am J Pathol. 1991 Jan;138(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- Tidball J. G., Lin C. Structural changes at the myogenic cell surface during the formation of myotendinous junctions. Cell Tissue Res. 1989 Jul;257(1):77–84. doi: 10.1007/BF00221636. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., O'Halloran T., Burridge K. Talin at myotendinous junctions. J Cell Biol. 1986 Oct;103(4):1465–1472. doi: 10.1083/jcb.103.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J. G., Quan D. M. Reduction in myotendinous junction surface area of rats subjected to 4-day spaceflight. J Appl Physiol (1985) 1992 Jul;73(1):59–64. doi: 10.1152/jappl.1992.73.1.59. [DOI] [PubMed] [Google Scholar]
- Tidball J. G. The geometry of actin filament-membrane associations can modify adhesive strength of the myotendinous junction. Cell Motil. 1983;3(5-6):439–447. doi: 10.1002/cm.970030512. [DOI] [PubMed] [Google Scholar]
- Trotter J. A., Eberhard S., Samora A. Structural domains of the muscle-tendon junction. 1. The internal lamina and the connecting domain. Anat Rec. 1983 Dec;207(4):573–591. doi: 10.1002/ar.1092070406. [DOI] [PubMed] [Google Scholar]
- Turner C. E., Kramarcy N., Sealock R., Burridge K. Localization of paxillin, a focal adhesion protein, to smooth muscle dense plaques, and the myotendinous and neuromuscular junctions of skeletal muscle. Exp Cell Res. 1991 Feb;192(2):651–655. doi: 10.1016/0014-4827(91)90090-h. [DOI] [PubMed] [Google Scholar]
- Turner P. R., Fong P. Y., Denetclaw W. F., Steinhardt R. A. Increased calcium influx in dystrophic muscle. J Cell Biol. 1991 Dec;115(6):1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Westwood T., Regen C. M., Steinhardt R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988 Oct 20;335(6192):735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Cullen M. J. A qualitative and quantitative study of the ultrastructure of regenerating muscle fibres in Duchenne muscular dystrophy and polymyositis. J Neurol Sci. 1987 Dec;82(1-3):181–192. doi: 10.1016/0022-510x(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A. P., Blau H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988 Feb 26;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Weller B., Karpati G., Carpenter S. Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J Neurol Sci. 1990 Dec;100(1-2):9–13. doi: 10.1016/0022-510x(90)90005-8. [DOI] [PubMed] [Google Scholar]
- Zamora A. J., Marini J. F. Tendon and myo-tendinous junction in an overloaded skeletal muscle of the rat. Anat Embryol (Berl) 1988;179(1):89–96. doi: 10.1007/BF00305103. [DOI] [PubMed] [Google Scholar]