Abstract
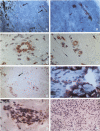
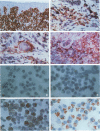
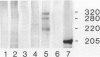
Recently, we have described a monoclonal antibody, named MS-1, which identifies a novel high-molecular-weight protein expressed by noncontinuous, sinusoidal endothelia and by interstitial dendritic cells in certain normal human organs (S Goerdt, LJ Walsh, GF Murphy, JS Pober, J Cell Biol 1991, 113:1425-1437; and LJ Walsh, S Goerdt, JS Pober, H Sueki, GF Murphy, Lab Invest 1991, 65: 732-741). In this report, we demonstrate in studying a variety of skin lesions that MS-1 antigen can also be expressed by endothelia of continuous origin under certain pathological conditions. Among the skin lesions tested, MS-1 antigen expression by endothelial cells of continuous origin is frequently observed in wound healing tissue, in cutaneous T-cell lymphoma, in psoriasis, and in melanoma metastasis, ie, in 100%, 80%, 71%, and 71% of cases, respectively. In contrast, endothelial MS-1 antigen expression rarely occurred in other skin lesions, including vascular tumors, six of which were Kaposi's sarcomas (13% and 0% of cases with vascular MS-1 expression, respectively). The percentage of cases with MS-1+ vessels is only marginally different in malignant versus benign lesions (55% versus 31%); when melanocytic nevi, primary melanomas, and melanoma metastases are compared, however, an increase in the percentage of cases with MS-1+ vessels is seen (31%, 50%, and 71%, respectively). Apart from wound healing, the relative number of MS-1+ vessels in a given lesion amounts to less than 5% compared with the number of continuous type vessels stained by monoclonal antibody 1F10 (S Goerdt, F Steckel, K Schulze-Osthoff, H-H Hagemeier, E Macher, C Sorg, Exp Cell Biol 1989, 57: 185-192). In addition, the occurrence of MS-1+ vessels is not related to the overall vascularity of a given lesion. Thus, the conditions for MS-1 antigen expression by endothelia of continuous origin cannot as yet be exactly defined. Furthermore, we have noticed that the number of MS-1+ dendritic cells varies considerably in skin lesions; in the early patch lesions of Kaposi's sarcoma and in juvenile xanthogranuloma MS-1+ cells even constitute the major cell type. This prompted us to investigate MS-1 antigen expression and its regulation in cultured human monocytes/macrophages. Expression of MS-1 antigen by these cells regularly starts at day 3 of culture and reaches its maximal value at day 9, after which it declines.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



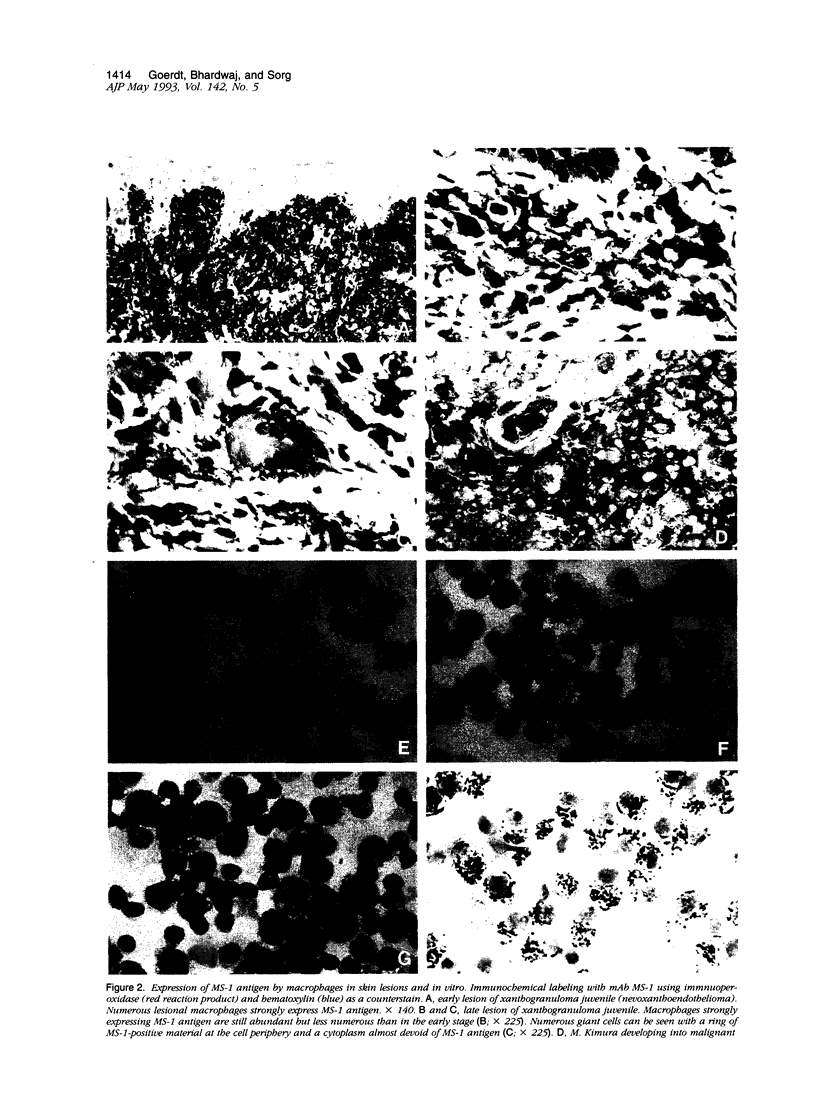

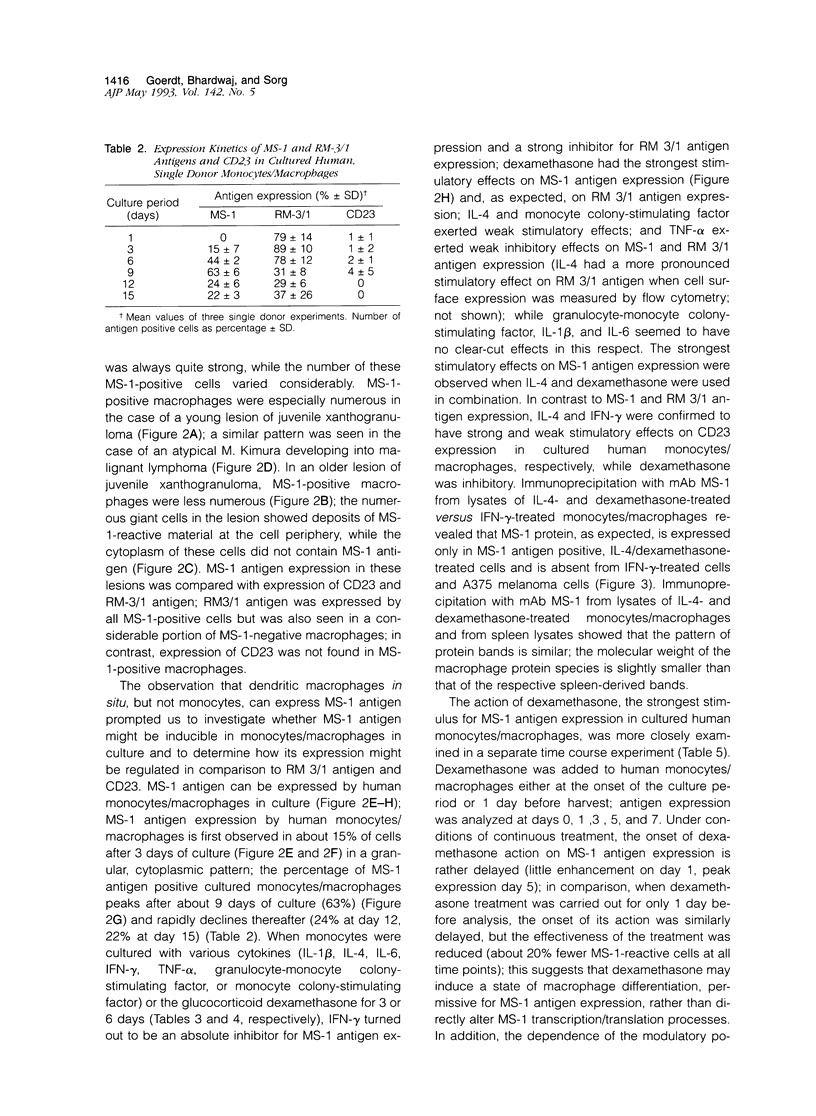
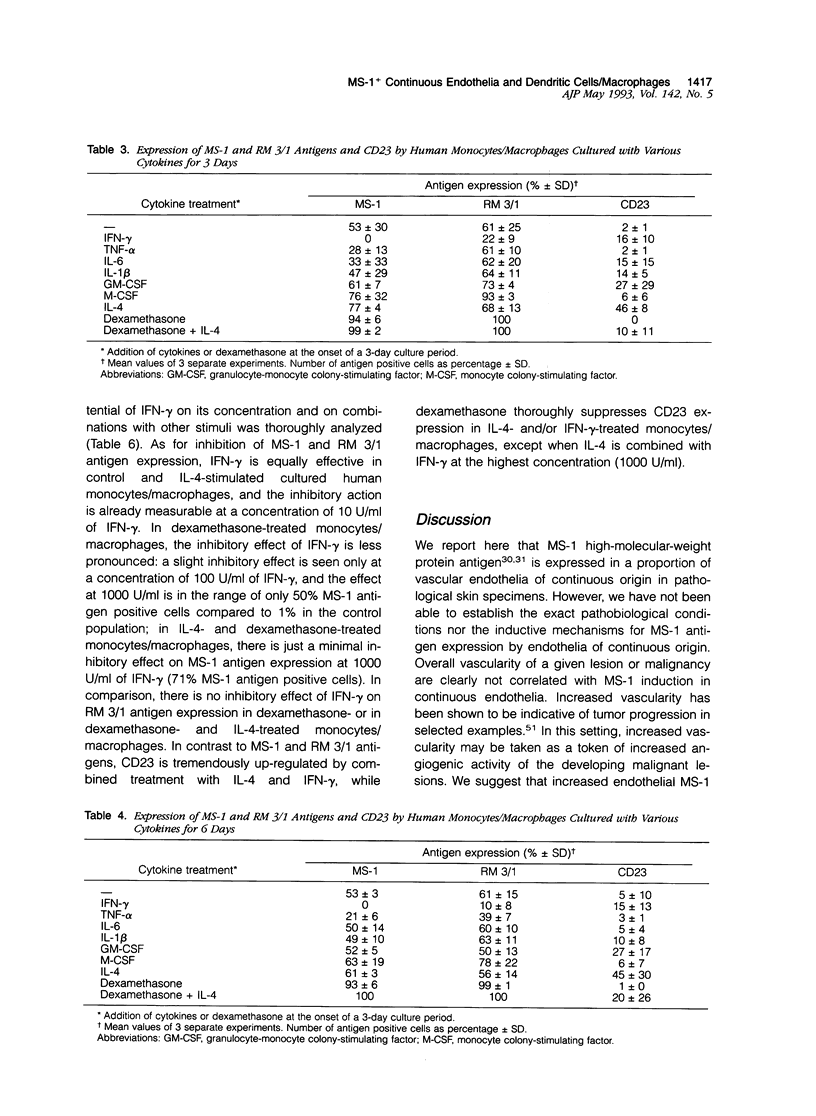
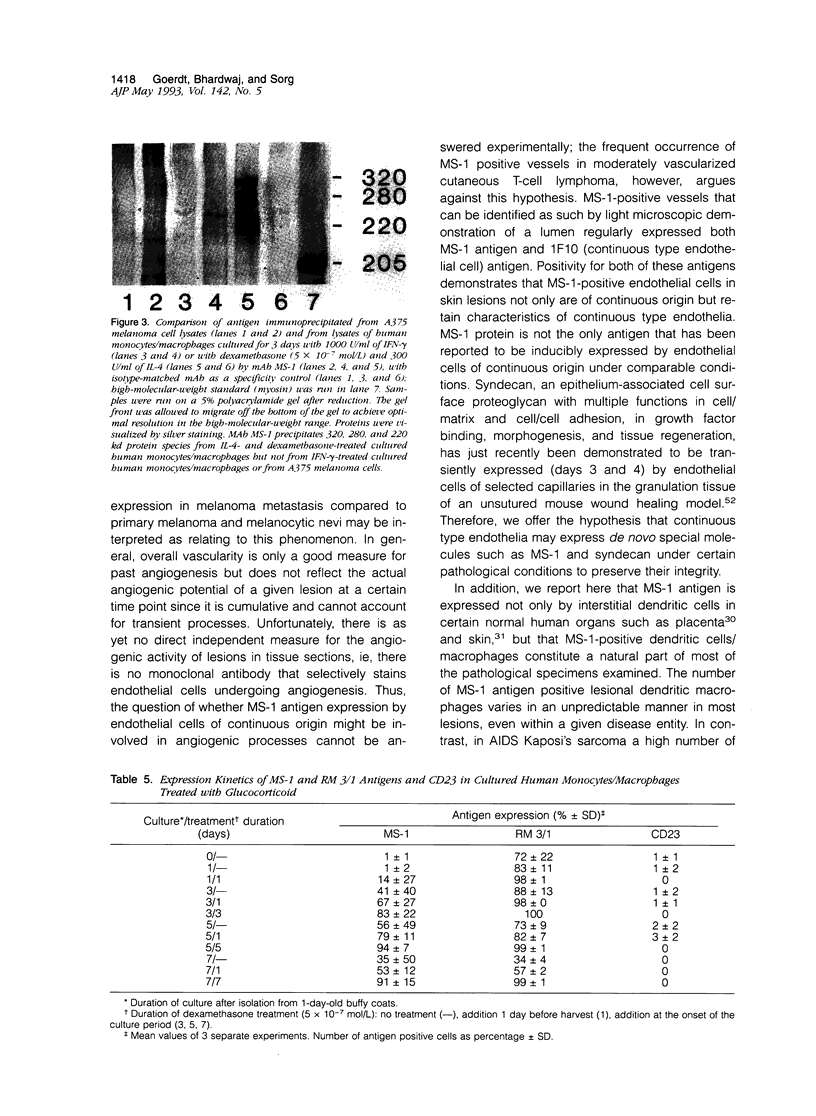




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman N. B., Hechmer P. A. Studies on the capillary permeability of experimental liver metastases. Surg Gynecol Obstet. 1978 Jun;146(6):884–888. [PubMed] [Google Scholar]
- Adány R., Glukhova M. A., Kabakov A. Y., Muszbek L. Characterisation of connective tissue cells containing factor XIII subunit a. J Clin Pathol. 1988 Jan;41(1):49–56. doi: 10.1136/jcp.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Muller W. A., Buck C. A., Newman P. J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991 Sep;114(5):1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese Estrada J., Piérard G. E. Factor-XIIIa-positive dendrocytes and the dermal microvascular unit. Dermatologica. 1990;180(1):51–53. doi: 10.1159/000247986. [DOI] [PubMed] [Google Scholar]
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beschorner W. E., Civin C. I., Strauss L. C. Localization of hematopoietic progenitor cells in tissue with the anti-My-10 monoclonal antibody. Am J Pathol. 1985 Apr;119(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Bieber T., Delespesse G. Gamma-interferon promotes the release of IgE-binding factors (soluble CD23) by human epidermal Langerhans cells. J Invest Dermatol. 1991 Sep;97(3):600–603. doi: 10.1111/1523-1747.ep12481944. [DOI] [PubMed] [Google Scholar]
- Bonnefoy J. Y., Aubry J. P., Peronne C., Wijdenes J., Banchereau J. Production and characterization of a monoclonal antibody specific for the human lymphocyte low affinity receptor for IgE: CD 23 is a low affinity receptor for IgE. J Immunol. 1987 May 1;138(9):2970–2978. [PubMed] [Google Scholar]
- Braverman I. M., Sibley J. Role of the microcirculation in the treatment and pathogenesis of psoriasis. J Invest Dermatol. 1982 Jan;78(1):12–17. doi: 10.1111/1523-1747.ep12497850. [DOI] [PubMed] [Google Scholar]
- Buckley P. J., Dickson S. A., Walker W. S. Human splenic sinusoidal lining cells express antigens associated with monocytes, macrophages, endothelial cells, and T lymphocytes. J Immunol. 1985 Apr;134(4):2310–2315. [PubMed] [Google Scholar]
- Cerio R., Griffiths C. E., Cooper K. D., Nickoloff B. J., Headington J. T. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol. 1989 Oct;121(4):421–431. doi: 10.1111/j.1365-2133.1989.tb15509.x. [DOI] [PubMed] [Google Scholar]
- Cheung D. L., Hart P. H., Vitti G. F., Whitty G. A., Hamilton J. A. Contrasting effects of interferon-gamma and interleukin-4 on the interleukin-6 activity of stimulated human monocytes. Immunology. 1990 Sep;71(1):70–75. [PMC free article] [PubMed] [Google Scholar]
- Crowley M. T., Inaba K., Witmer-Pack M. D., Gezelter S., Steinman R. M. Use of the fluorescence activated cell sorter to enrich dendritic cells from mouse spleen. J Immunol Methods. 1990 Oct 4;133(1):55–66. doi: 10.1016/0022-1759(90)90318-p. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delespesse G., Sarfati M. An update on human CD23 (Fc epsilon RII). Fc epsilon RII and IgE-BFs (soluble CD23) play an essential role in the regulation of human IgE synthesis. Clin Exp Allergy. 1991 Jan;21 (Suppl 1):153–161. doi: 10.1111/j.1365-2222.1991.tb01720.x. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Leaky tumor vessels: consequences for tumor stroma generation and for solid tumor therapy. Prog Clin Biol Res. 1990;354A:317–330. [PubMed] [Google Scholar]
- Dvorak H. F., Sioussat T. M., Brown L. F., Berse B., Nagy J. A., Sotrel A., Manseau E. J., Van de Water L., Senger D. R. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991 Nov 1;174(5):1275–1278. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K., Vainio S., Laato M., Salmivirta M., Thesleff I., Jalkanen M. Induced expression of syndecan in healing wounds. J Cell Biol. 1991 Aug;114(3):585–595. doi: 10.1083/jcb.114.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fear J. D., Jackson P., Gray C., Miloszewski K. J., Losowsky M. S. Localisation of factor XIII in human tissues using an immunoperoxidase technique. J Clin Pathol. 1984 May;37(5):560–563. doi: 10.1136/jcp.37.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina L., Molgaard H. V., Robertson D., Bradley N. J., Monaghan P., Delia D., Sutherland D. R., Baker M. A., Greaves M. F. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990 Jun 15;75(12):2417–2426. [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerdt S., Steckel F., Schulze-Osthoff K., Hagemeier H. H., Macher E., Sorg C. Characterization and differential expression of an endothelial cell-specific surface antigen in continuous and sinusoidal endothelial, in skin vascular lesions and in vitro. Exp Cell Biol. 1989;57(4):185–192. doi: 10.1159/000163524. [DOI] [PubMed] [Google Scholar]
- Goerdt S., Walsh L. J., Murphy G. F., Pober J. S. Identification of a novel high molecular weight protein preferentially expressed by sinusoidal endothelial cells in normal human tissues. J Cell Biol. 1991 Jun;113(6):1425–1437. doi: 10.1083/jcb.113.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkiss G. D., Hopkins J., McConnell I. Uptake of antigen by afferent lymph dendritic cells mediated by antibody. Eur J Immunol. 1990 Nov;20(11):2367–2373. doi: 10.1002/eji.1830201102. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Whitty G. A., Burgess D. R., Croatto M., Hamilton J. A. Augmentation of glucocorticoid action on human monocytes by interleukin-4. Lymphokine Res. 1990 Summer;9(2):147–153. [PubMed] [Google Scholar]
- Heimark R. L., Degner M., Schwartz S. M. Identification of a Ca2(+)-dependent cell-cell adhesion molecule in endothelial cells. J Cell Biol. 1990 May;110(5):1745–1756. doi: 10.1083/jcb.110.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. L., Holbrook K. A. Development of human embryonic and fetal dermal vasculature. J Invest Dermatol. 1989 Aug;93(2 Suppl):10S–17S. doi: 10.1111/1523-1747.ep12580896. [DOI] [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Lew W., Oppenheim J. J., Matsushima K. Analysis of the suppression of IL-1 alpha and IL-1 beta production in human peripheral blood mononuclear adherent cells by a glucocorticoid hormone. J Immunol. 1988 Mar 15;140(6):1895–1902. [PubMed] [Google Scholar]
- Lobb R. R., Key M. E., Alderman E. M., Fett J. W. Partial purification and characterization of a vascular permeability factor secreted by a human colon adenocarcinoma cell line. Int J Cancer. 1985 Oct 15;36(4):473–478. doi: 10.1002/ijc.2910360410. [DOI] [PubMed] [Google Scholar]
- Manasek F. J. The ultrastructure of embryonic myocardial blood vessels. Dev Biol. 1971 Sep;26(1):42–54. doi: 10.1016/0012-1606(71)90106-0. [DOI] [PubMed] [Google Scholar]
- Marianayagam L., Poulter L. W. Corticosteroid can alter antigen expression on alveolar macrophages. Clin Exp Immunol. 1991 Sep;85(3):531–535. doi: 10.1111/j.1365-2249.1991.tb05762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes A., Rennick D. M. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988 Feb 1;167(2):598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Fletcher V., Conant M. A. Early lesions of Kaposi's sarcoma in homosexual men. An ultrastructural comparison with other vascular proliferations in skin. Am J Pathol. 1983 Apr;111(1):62–77. [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Ratti C. M., McDonnell S. L., Cohn Z. A. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989 Aug 1;170(2):399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro C. S., Campbell D. A., Collings L. A., Poulter L. W. Monoclonal antibodies distinguish macrophages and epithelioid cells in sarcoidosis and leprosy. Clin Exp Immunol. 1987 May;68(2):282–287. [PMC free article] [PubMed] [Google Scholar]
- Murphy G. F., Bronstein B. R., Knowles R. W., Bhan A. K. Ultrastructural documentation of M241 glycoprotein on dendritic and endothelial cells in normal human skin. Lab Invest. 1985 Mar;52(3):264–269. [PubMed] [Google Scholar]
- Nagura H., Koshikawa T., Fukuda Y., Asai J. Hepatic vascular endothelial cells heterogenously express surface antigens associated with monocytes, macrophages and T lymphocytes. Virchows Arch A Pathol Anat Histopathol. 1986;409(4):407–416. doi: 10.1007/BF00705413. [DOI] [PubMed] [Google Scholar]
- Nemes Z., Thomázy V., Adány R., Muszbek L. Identification of histiocytic reticulum cells by the immunohistochemical demonstration of factor XIII (F-XIIIa) in human lymph nodes. J Pathol. 1986 Jun;149(2):121–132. doi: 10.1002/path.1711490207. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Griffiths C. E. Factor XIIIa-expressing dermal dendrocytes in AIDS-associated cutaneous Kaposi's sarcomas. Science. 1989 Mar 31;243(4899):1736–1737. doi: 10.1126/science.2564703. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J. The human progenitor cell antigen (CD34) is localized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Kaposi's sarcoma. Arch Dermatol. 1991 Apr;127(4):523–529. [PubMed] [Google Scholar]
- Nishio S., Ohta M., Abe M., Kitamura K. Microvascular abnormalities in ethylnitrosourea (ENU)-induced rat brain tumors: structural basis for altered blood-brain barrier function. Acta Neuropathol. 1983;59(1):1–10. doi: 10.1007/BF00690311. [DOI] [PubMed] [Google Scholar]
- Novak R. M., Holzer T. J., Kennedy M. M., Heynen C. A., Dawson G. The effect of interleukin 4 (BSF-1) on infection of peripheral blood monocyte-derived macrophages with HIV-1. AIDS Res Hum Retroviruses. 1990 Aug;6(8):973–976. doi: 10.1089/aid.1990.6.973. [DOI] [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Hansmann M. L., Peters K. P. Monoclonal antibody Ki-M4 specifically recognizes human dendritic reticulum cells (follicular dendritic cells) and their possible precursor in blood. Blood. 1983 Sep;62(3):585–590. [PubMed] [Google Scholar]
- Penneys N. S. Factor XIII expression in the skin: observations and a hypothesis. J Am Acad Dermatol. 1990 Mar;22(3):484–488. doi: 10.1016/0190-9622(90)70068-s. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Rutgers J. L., Wieczorek R., Bonetti F., Kaplan K. L., Posnett D. N., Friedman-Kien A. E., Knowles D. M., 2nd The expression of endothelial cell surface antigens by AIDS-associated Kaposi's sarcoma. Evidence for a vascular endothelial cell origin. Am J Pathol. 1986 Mar;122(3):493–499. [PMC free article] [PubMed] [Google Scholar]
- SCHOEFL G. I. STUDIES ON INFLAMMATION. III. GROWING CAPILLARIES: THEIR STRUCTURE AND PERMEABILITY. Virchows Arch Pathol Anat Physiol Klin Med. 1963 Nov 8;337:97–141. [PubMed] [Google Scholar]
- Sankey E. A., More L., Dhillon A. P. QBEnd/10: a new immunostain for the routine diagnosis of Kaposi's sarcoma. J Pathol. 1990 Jul;161(3):267–271. doi: 10.1002/path.1711610315. [DOI] [PubMed] [Google Scholar]
- Schaffner A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest. 1985 Nov;76(5):1755–1764. doi: 10.1172/JCI112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., de Waal R. M., Bradley N. J., Skene A. I., Davies A. J., Greaves M. F., Denekamp J., Ruiter D. J. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990 Jun;62(6):690–696. [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Spiteri M. A., Poulter L. W. Characterization of immune inducer and suppressor macrophages from the normal human lung. Clin Exp Immunol. 1991 Jan;83(1):157–162. doi: 10.1111/j.1365-2249.1991.tb05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl G. Dendritic cells of the skin. Dermatol Clin. 1990 Oct;8(4):673–679. [PubMed] [Google Scholar]
- Vercelli D., Jabara H. H., Lee B. W., Woodland N., Geha R. S., Leung D. Y. Human recombinant interleukin 4 induces Fc epsilon R2/CD23 on normal human monocytes. J Exp Med. 1988 Apr 1;167(4):1406–1416. doi: 10.1084/jem.167.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide-stimulated human monocytes. Immunology. 1988 Feb;63(2):299–302. [PMC free article] [PubMed] [Google Scholar]
- Wacker H. H., Radzun H. J., Mielke V., Parwaresch M. R. Selective recognition of rat follicular dendritic cells (dendritic reticulum cells) by a new monoclonal antibody Ki-M4R in vitro and in vivo. J Leukoc Biol. 1987 Jan;41(1):70–77. doi: 10.1002/jlb.41.1.70. [DOI] [PubMed] [Google Scholar]
- Walsh L. J., Goerdt S., Pober J. S., Sueki H., Murphy G. F. MS-1 sinusoidal endothelial antigen is expressed by factor XIIIa+, HLA-DR+ dermal perivascular dendritic cells. Lab Invest. 1991 Dec;65(6):732–741. [PubMed] [Google Scholar]
- Ward J. D., Hadfield M. G., Becker D. P., Lovings E. T. Endothelial fenestrations and other vascular alterations in primary melanoma of the central nervous system. Cancer. 1974 Dec;34(6):1982–1991. doi: 10.1002/1097-0142(197412)34:6<1982::aid-cncr2820340617>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Weber K., Braun-Falco O. Ultrastructure of blood vessels in human granulation tissue. Arch Dermatol Forsch. 1973;248(1):29–44. doi: 10.1007/BF00594710. [DOI] [PubMed] [Google Scholar]
- Weidner N., Semple J. P., Welch W. R., Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991 Jan 3;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Hobbs M. M., Misukonis M. A. Recombinant human gamma-interferon induces human monocyte polykaryon formation. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4554–4557. doi: 10.1073/pnas.81.14.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. Biochemical actions of glucocorticoids on macrophages in culture. Specific inhibition of elastase, collagenase, and plasminogen activator secretion and effects on other metabolic functions. J Exp Med. 1978 Jun 1;147(6):1695–1712. doi: 10.1084/jem.147.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Ushio Y., Hayakawa T., Kato A., Yamada N., Mogami H. Quantitative autoradiographic measurements of blood-brain barrier permeability in the rat glioma model. J Neurosurg. 1982 Sep;57(3):394–398. doi: 10.3171/jns.1982.57.3.0394. [DOI] [PubMed] [Google Scholar]
- Yokota A., Kikutani H., Tanaka T., Sato R., Barsumian E. L., Suemura M., Kishimoto T. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988 Nov 18;55(4):611–618. doi: 10.1016/0092-8674(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Zwadlo-Klarwasser G., Bent S., Haubeck H. D., Sorg C., Schmutzler W. Glucocorticoid-induced appearance of the macrophage subtype RM 3/1 in peripheral blood of man. Int Arch Allergy Appl Immunol. 1990;91(2):175–180. doi: 10.1159/000235111. [DOI] [PubMed] [Google Scholar]
- Zwadlo G., Voegeli R., Schulze Osthoff K., Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55(6):295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- te Velde A. A., Rousset F., Peronne C., De Vries J. E., Figdor C. G. IFN-alpha and IFN-gamma have different regulatory effects on IL-4-induced membrane expression of Fc epsilon RIIb and release of soluble Fc epsilon RIIb by human monocytes. J Immunol. 1990 Apr 15;144(8):3052–3059. [PubMed] [Google Scholar]