Abstract
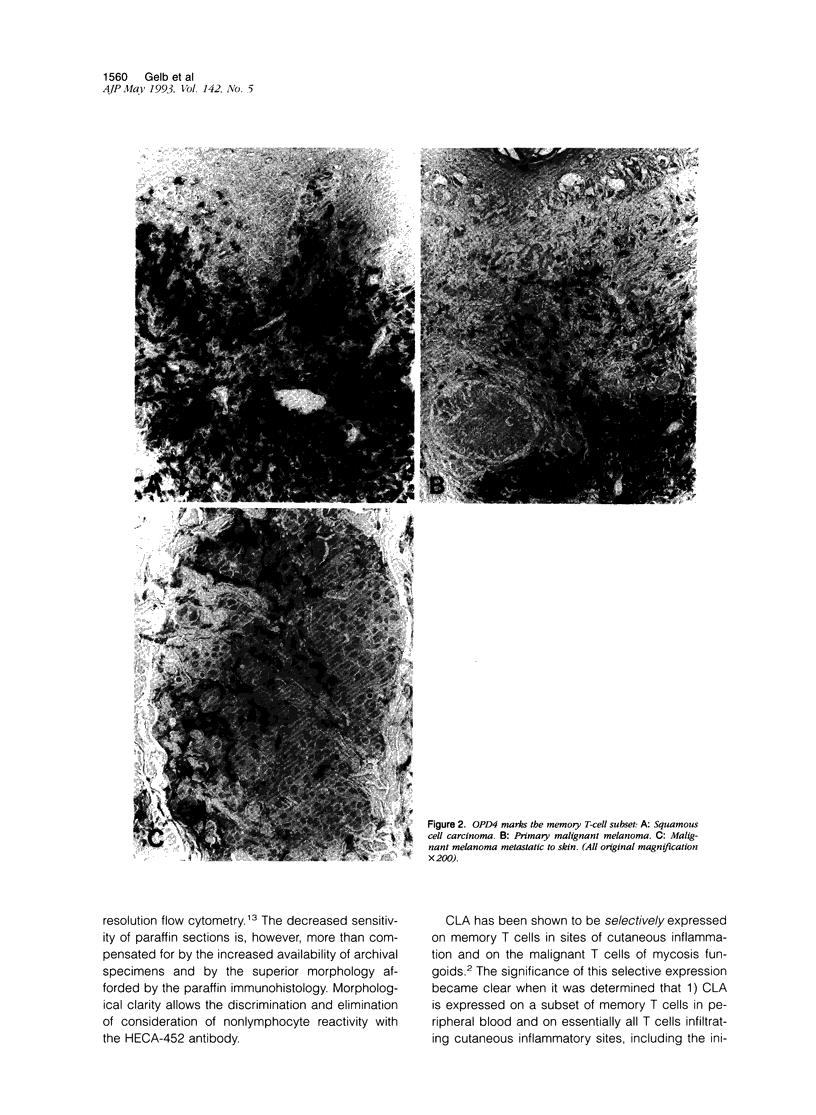
The cutaneous lymphocyte-associated antigen (CLA) is the T-cell ligand for E-selectin and is involved in tissue selective migration of memory/effector T cells to chronic inflammatory sites in skin. Here, we examine the hypothesis that CLA is also involved in the local host immune response to cutaneous neoplasms. Eleven primary cutaneous melanomas, nine primary cutaneous squamous cell carcinomas, and 11 assorted neoplasms metastatic to cutaneous and noncutaneous sites were immunostained with anti-CLA (HECA-452), as well as antibodies directed against B cells (CD20), T/NK cells (CD43), and memory/effector T cells (CD45RO). Essentially all of the lymphocytes surrounding and infiltrating both the cutaneous and noncutaneous tumors were CD43+/CD20-, and most expressed the memory/effector marker CD45RO. CLA was expressed on 10 to 80% (mean: 50%) of T cells associated with primary cutaneous neoplasms (including both melanomas and squamous cell carcinomas) but was essentially absent from noncutaneous primaries (including those metastatic to dermis) and from cutaneous primaries metastatic to dermis or other sites. Overall, the results suggest that CLA+memory T cells are a major component of the local host immune response to cutaneous neoplasms and are likely recruited to the skin by site-specific rather than tumor-specific mechanisms. The lack of a CLA+T-cell response to dermal metastases suggests that epidermal involvement may be required to attract this subset.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailar J. C., 3rd Science, statistics, and deception. Ann Intern Med. 1986 Feb;104(2):259–260. doi: 10.7326/0003-4819-104-2-259. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991 Dec 1;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindl J. M., Warnke R. A. Advantages of detecting monoclonal antibody binding to tissue sections with biotin and avidin reagents in Coplin jars. Am J Clin Pathol. 1986 Apr;85(4):490–493. doi: 10.1093/ajcp/85.4.490. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Brousse N., Lisowska-Grospierre B., Guy-Grand D., Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987 Sep;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Hacene K., Le Doussal V., Brunet M., Lemoine F., Guerin P., Hebert H. Prognostic index for clinical Stage I cutaneous malignant melanoma. Cancer Res. 1983 Jun;43(6):2991–2996. [PubMed] [Google Scholar]
- Jarry A., Cerf-Bensussan N., Brousse N., Guy-Grand D., Muzeau F., Potet F. Same peculiar subset of HML1 + lymphocytes present within normal intestinal epithelium is associated with tumoral epithelium of gastrointestinal carcinomas. Gut. 1988 Dec;29(12):1632–1638. doi: 10.1136/gut.29.12.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem K., Rigney E., Cordell J., Simmons D., Stross P., Turley H., Seed B., Mason D. A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol. 1989 Sep;73(1):6–11. doi: 10.1111/j.1365-2141.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Mizutani H., Black R., Kupper T. S. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta. Different strategies of IL-1 production and processing in monocytes and keratinocytes. J Clin Invest. 1991 Mar;87(3):1066–1071. doi: 10.1172/JCI115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani H., Schechter N., Lazarus G., Black R. A., Kupper T. S. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med. 1991 Oct 1;174(4):821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Michie S. A., Rott L. S., Butcher E. C. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990 May;136(5):1053–1068. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Terstappen L. W., Rott L. S., Streeter P. R., Stein H., Butcher E. C. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990 Nov 15;145(10):3247–3255. [PubMed] [Google Scholar]
- Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Bergstresser P. R., Terstappen L. W. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol. 1993 Feb 1;150(3):1122–1136. [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Poppema S., Lai R., Visser L. Monoclonal antibody OPD4 is reactive with CD45RO, but differs from UCHL1 by the absence of monocyte reactivity. Am J Pathol. 1991 Oct;139(4):725–729. [PMC free article] [PubMed] [Google Scholar]
- Prade M., Bognel C., Charpentier P., Gadenne C., Duvillard P., Sancho-Garnier H., Petit J. Y. Malignant melanoma of the skin: prognostic factors derived from a multifactorial analysis of 239 cases. Am J Dermatopathol. 1982 Oct;4(5):411–412. [PubMed] [Google Scholar]
- Schieferdecker H. L., Ullrich R., Weiss-Breckwoldt A. N., Schwarting R., Stein H., Riecken E. O., Zeitz M. The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol. 1990 Apr 1;144(7):2541–2549. [PubMed] [Google Scholar]
- Shaw H. M., Balch C. M., Soong S. J., Milton G. W., McCarthy W. H. Prognostic histopathological factors in malignant melanoma. Pathology. 1985 Apr;17(2):271–274. doi: 10.3109/00313028509063766. [DOI] [PubMed] [Google Scholar]
- Van Der Esch E. P., Cascinelli N., Preda F., Morabito A., Bufalino R. Stage I melanoma of the skin: evaluation of prognosis according to histologic characteristics. Cancer. 1981 Oct 1;48(7):1668–1673. doi: 10.1002/1097-0142(19811001)48:7<1668::aid-cncr2820480732>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Vargas-Cortes M., Axelsson B., Larsson A., Berzins T., Perlmann P. Enhancement of human spontaneous cell-mediated cytotoxicity by a monoclonal antibody against the large sialoglycoprotein (CD 43) on peripheral blood lymphocytes. Scand J Immunol. 1988 Jun;27(6):661–671. doi: 10.1111/j.1365-3083.1988.tb02399.x. [DOI] [PubMed] [Google Scholar]
- Yoshino T., Mukuzono H., Aoki H., Takahashi K., Takeuchi T., Kubonishi I., Ohtsuki Y., Motoi M., Akagi T. A novel monoclonal antibody (OPD4) recognizing a helper/inducer T cell subset. Its application to paraffin-embedded tissues. Am J Pathol. 1989 Jun;134(6):1339–1346. [PMC free article] [PubMed] [Google Scholar]
- Yuan Q., Jiang W. M., Hollander D., Leung E., Watson J. D., Krissansen G. W. Identity between the novel integrin beta 7 subunit and an antigen found highly expressed on intraepithelial lymphocytes in the small intestine. Biochem Biophys Res Commun. 1991 May 15;176(3):1443–1449. doi: 10.1016/0006-291x(91)90448-g. [DOI] [PubMed] [Google Scholar]