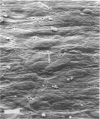
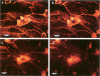
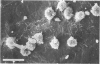
Abstract
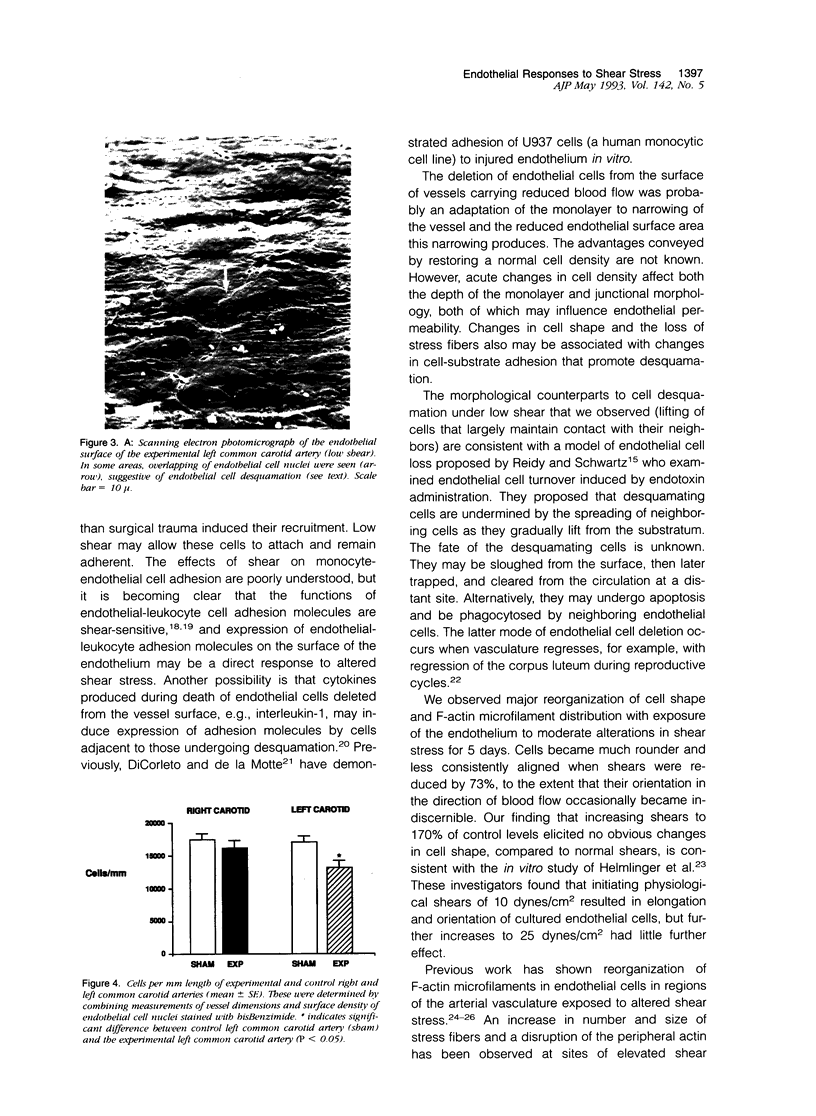
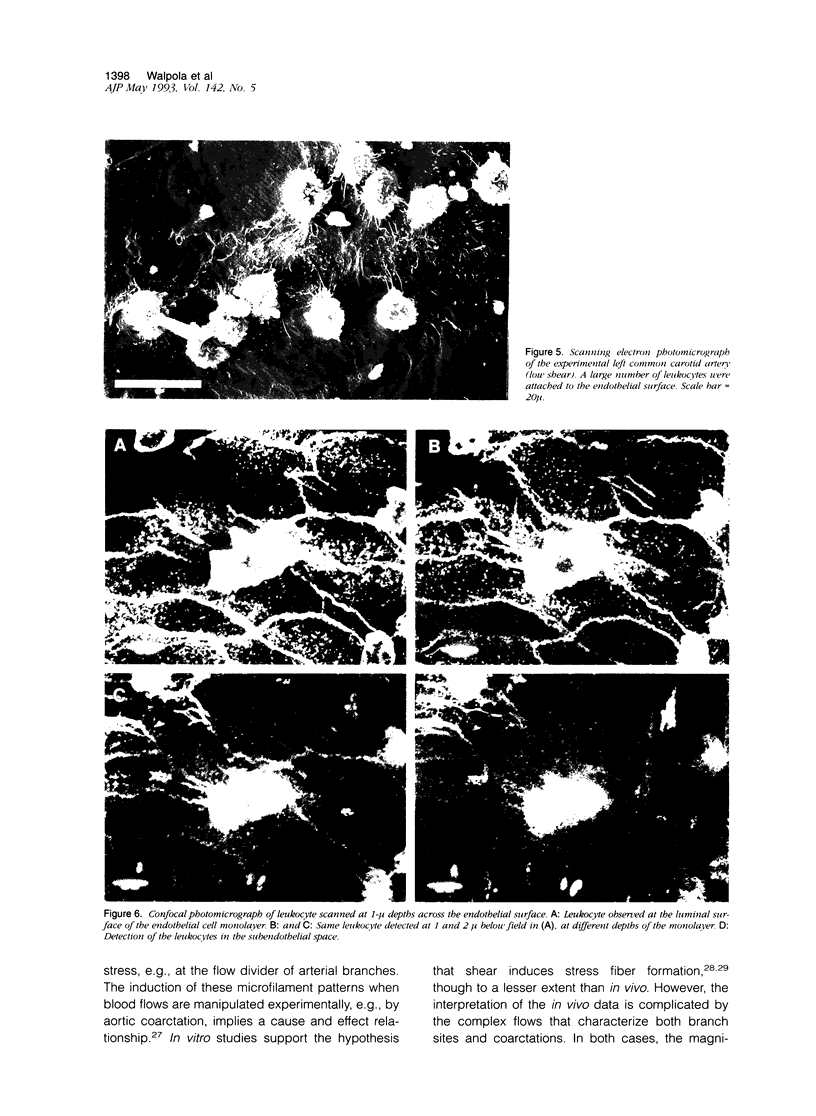
Left common carotid arteries of New Zealand white rabbits were ligated rostral to origin of the thyroid artery to reduce flow in the carotid upstream of this branch, and the vessels were examined 5 days later. Estimates of mean shear stress in the upstream carotid artery indicated a decrease of 73% (from 12.1 +/- 1.6 dynes/cm2 to 3.26 +/- 0.58 dynes/cm2). The contralateral common carotid artery carried collateral flow and experienced a 170% increase in shear stress (from 11.3 +/- 1.6 dynes/cm2 to 30.5 +/- 4.6 dynes/cm2). There was an adaptive reduction in the diameter in the left common carotid artery (low shear) from 2.07 +/- 0.06 mm to 1.75 +/- 0.12 mm, but the diameter of the right carotid was unchanged. Fluorescence microscopy and scanning electron microscopy of endothelium exposed to low shear revealed attachment of leukocytes (5.02 +/- 1.59 cells/mm2, mean +/- SE) that were identified as monocytes using the monoclonal antibody HAM 56. Laser confocal microscopy demonstrated that they were migrating across the endothelial cell monolayer. Fluorescence microscopy and scanning electron microscopy of left common carotid artery (low shear) also revealed cell morphology suggestive of endothelial cell desquamation. Endothelial cell loss was confirmed by morphometric determination of cell number (1.29 +/- 0.13 x 10(4) cells/mm length in experimental animals versus 1.71 +/- 0.08 x 10(4) cells/mm length in sham-operated animals). This endothelial cell loss may be an adaptation to a narrowing of carotid arteries exposed to low shear, which reduces luminal surface area of the vessel. Staining of F-actin with rhodamine phalloidin showed that endothelial cells exposed to low shear were less elongated and had fewer stress fibers than normal cells. By contrast, increasing shear stress by two- to threefold caused an increase in the number of stress fibers and a reduction in peripheral actin staining. Distal carotid ligation provided a consistent and well-defined in vivo technique for manipulating shear stresses imposed on a large population of endothelial cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura T., Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990 Apr;66(4):1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- Azmi T. I., O'Shea J. D. Mechanism of deletion of endothelial cells during regression of the corpus luteum. Lab Invest. 1984 Aug;51(2):206–217. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Molony L., Kelly T. Adhesion plaques: sites of transmembrane interaction between the extracellular matrix and the actin cytoskeleton. J Cell Sci Suppl. 1987;8:211–229. doi: 10.1242/jcs.1987.supplement_8.12. [DOI] [PubMed] [Google Scholar]
- Caro C. G., Fitz-Gerald J. M., Schroter R. C. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci. 1971 Feb 16;177(1046):109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- Cornhill J. F., Barrett W. A., Herderick E. E., Mahley R. W., Fry D. L. Topographic study of sudanophilic lesions in cholesterol-fed minipigs by image analysis. Arteriosclerosis. 1985 Sep-Oct;5(5):415–426. doi: 10.1161/01.atv.5.5.415. [DOI] [PubMed] [Google Scholar]
- Cornhill J. F., Roach M. R. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976 May-Jun;23(3):489–501. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., de la Motte C. A. Characterization of the adhesion of the human monocytic cell line U937 to cultured endothelial cells. J Clin Invest. 1985 Apr;75(4):1153–1161. doi: 10.1172/JCI111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke R. P., Gräfe M., Schnittler H., Seiffge D., Mittermayer C., Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984 Feb 16;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Fry D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968 Feb;22(2):165–197. doi: 10.1161/01.res.22.2.165. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Glagov S., Zarins C., Giddens D. P., Ku D. N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988 Oct;112(10):1018–1031. [PubMed] [Google Scholar]
- Helmlinger G., Geiger R. V., Schreck S., Nerem R. M. Effects of pulsatile flow on cultured vascular endothelial cell morphology. J Biomech Eng. 1991 May;113(2):123–131. doi: 10.1115/1.2891226. [DOI] [PubMed] [Google Scholar]
- Houle S., Roach M. R. Flow studies in a rigid model of an aorto-renal junction. A case for high shear as a cause of the localization of sudanophilic lesions in rabbits. Atherosclerosis. 1981 Nov-Dec;40(3-4):231–244. doi: 10.1016/0021-9150(81)90133-7. [DOI] [PubMed] [Google Scholar]
- Kamiya A., Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980 Jul;239(1):H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Kim D. W., Gotlieb A. I., Langille B. L. In vivo modulation of endothelial F-actin microfilaments by experimental alterations in shear stress. Arteriosclerosis. 1989 Jul-Aug;9(4):439–445. doi: 10.1161/01.atv.9.4.439. [DOI] [PubMed] [Google Scholar]
- Kim D. W., Langille B. L., Wong M. K., Gotlieb A. I. Patterns of endothelial microfilament distribution in the rabbit aorta in situ. Circ Res. 1989 Jan;64(1):21–31. doi: 10.1161/01.res.64.1.21. [DOI] [PubMed] [Google Scholar]
- Ku D. N., Giddens D. P., Zarins C. K., Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985 May-Jun;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Smith C. W., Eskin S. G., McIntire L. V. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990 Jan 1;75(1):227–237. [PubMed] [Google Scholar]
- Masuda H., Kawamura K., Tohda K., Shozawa T., Sageshima M., Kamiya A. Increase in endothelial cell density before artery enlargement in flow-loaded canine carotid artery. Arteriosclerosis. 1989 Nov-Dec;9(6):812–823. doi: 10.1161/01.atv.9.6.812. [DOI] [PubMed] [Google Scholar]
- Montenegro M. R., Eggen D. A. Topography of atherosclerosis in the coronary arteries. Lab Invest. 1968 May;18(5):586–593. [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial injury and regeneration. IV. Endotoxin: a nondenuding injury to aortic endothelium. Lab Invest. 1983 Jan;48(1):25–34. [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Sinzinger H., Silberbauer K., Auerswald W. Quantitative investigation of sudanophilic lesions around the aortic ostia of human fetuses, newborn and children. Blood Vessels. 1980;17(1):44–52. doi: 10.1159/000158233. [DOI] [PubMed] [Google Scholar]
- Strauss B. I., Langille B. L., Gotlieb A. I. In situ localization of F-actin microfilaments in the vasculature of the porcine retina. Exp Eye Res. 1987 Oct;45(4):533–544. doi: 10.1016/s0014-4835(87)80064-7. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Rosenfeld M., Ross R., Gown A. M. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986 Nov-Dec;6(6):601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- Wechezak A. R., Viggers R. F., Sauvage L. R. Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear stress. Lab Invest. 1985 Dec;53(6):639–647. [PubMed] [Google Scholar]
- Wechezak A. R., Wight T. N., Viggers R. F., Sauvage L. R. Endothelial adherence under shear stress is dependent upon microfilament reorganization. J Cell Physiol. 1989 Apr;139(1):136–146. doi: 10.1002/jcp.1041390120. [DOI] [PubMed] [Google Scholar]
- White G. E., Fujiwara K. Expression and intracellular distribution of stress fibers in aortic endothelium. J Cell Biol. 1986 Jul;103(1):63–70. doi: 10.1083/jcb.103.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. E., Gimbrone M. A., Jr, Fujiwara K. Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J Cell Biol. 1983 Aug;97(2):416–424. doi: 10.1083/jcb.97.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand T., Majno G., Nunnari J. J., Hoffman A. H., Savilonis B. J., MacWilliams B., Joris I. Lipid deposition and intimal stress and strain. A study in rats with aortic stenosis. Am J Pathol. 1991 Jul;139(1):101–113. [PMC free article] [PubMed] [Google Scholar]
- Zarins C. K., Zatina M. A., Giddens D. P., Ku D. N., Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987 Mar;5(3):413–420. [PubMed] [Google Scholar]