Abstract
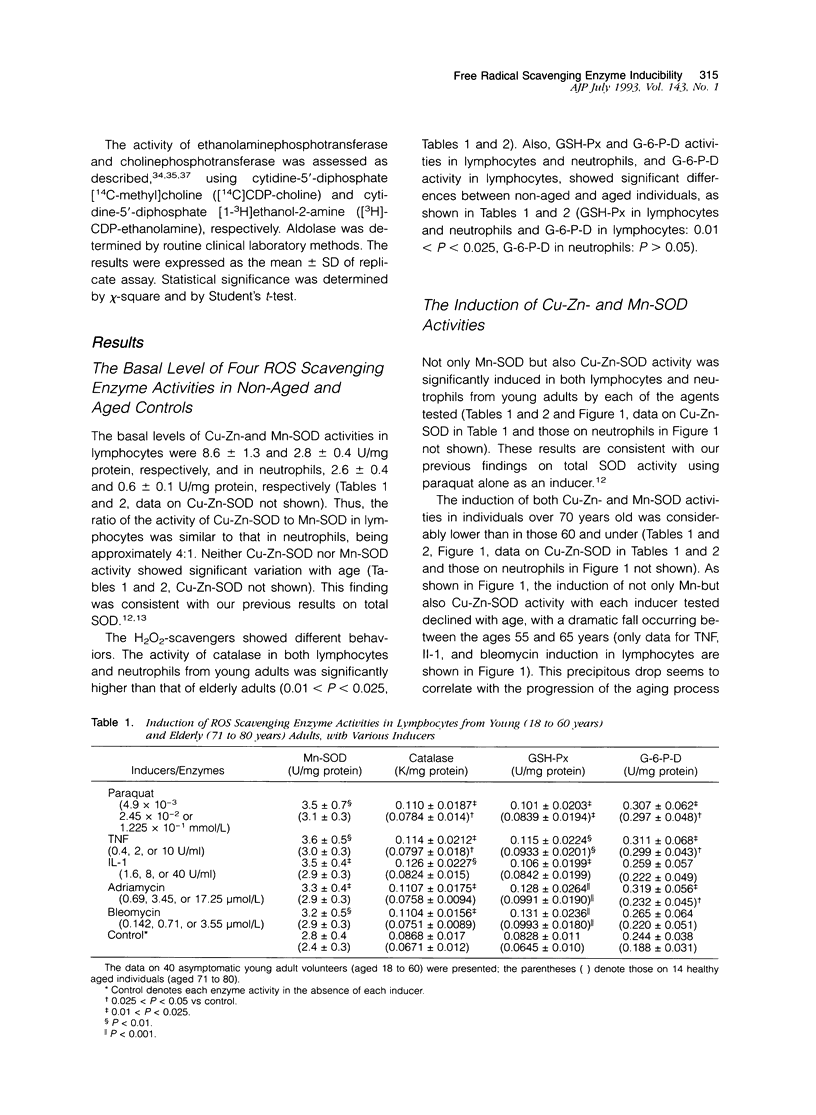
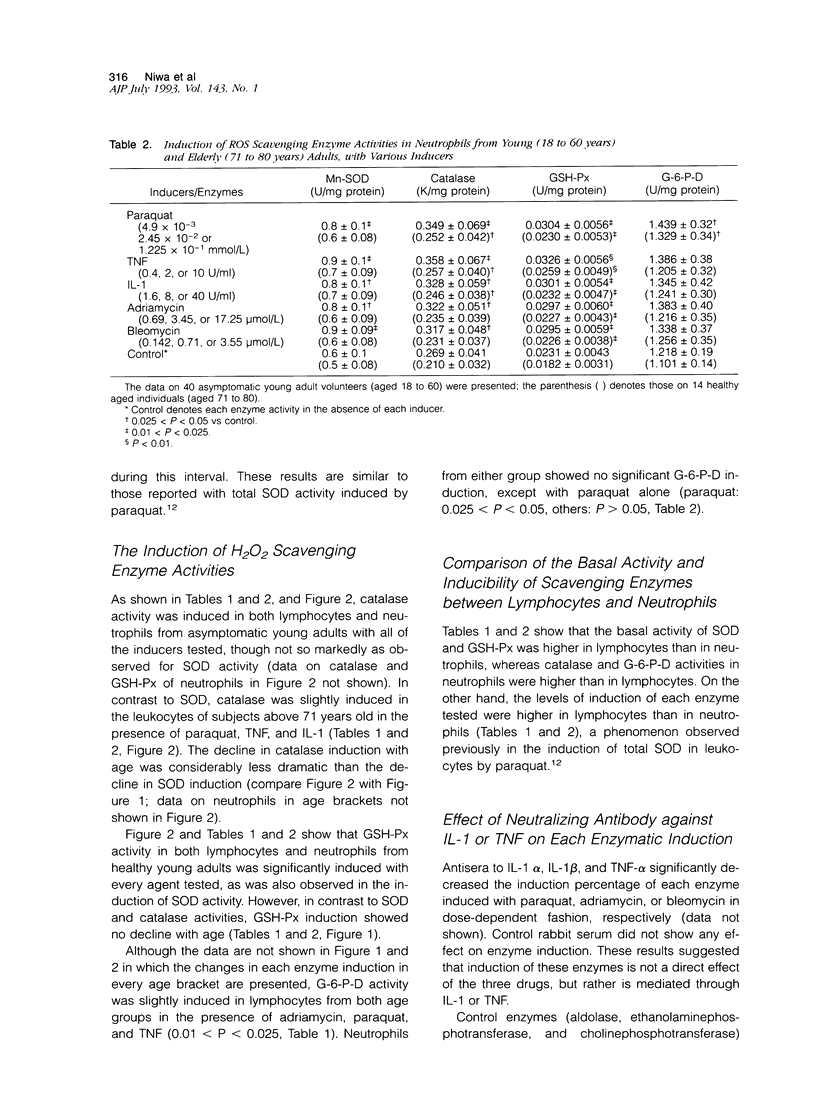
Several enzymes, including superoxide dismutase (SOD), catalase, glutathione peroxidase, and D-glucose-6-phosphate dehydrogenase are capable of scavenging reactive oxygen species in in vivo. We assessed both basal levels and the capacity of these enzyme activities to be induced in human leukocytes in response to a variety of agents. Basal activity of copper-zinc SOD, and manganese SOD showed little variation with age. In contrast, the basal activity of the three H2O2 scavenging enzymes, catalase, glutathione peroxidase, and D-glucose-6-phosphate dehydrogenase, was significantly higher in younger adults than in elderly individuals. Both manganese SOD and copper, zinc SOD activities were significantly induced by paraquat, interleukin-1, tumor necrosis factor, adriamycin, and bleomycin in lymphocytes and neutrophils from asymptomatic non-aged adults, whereas neither activity was induced in aged individuals. In contrast, glutathione peroxidase activity was significantly induced in both groups of subjects, whereas catalase and D-glucose-6-phosphate dehydrogenase were only slightly induced in either. Enzyme induction with paraquat, adriamycin, or bleomycin was inhibitable by neutralizing antibody to interleukin-1 and tumor necrosis factor, suggesting that the inductions observed with these three drugs are due to the distal mediators, interleukin-1 or tumor necrosis factor released from the cells. Finally, as observed in the regulation of genes in eukaryotes (Storz et al: Bacterial defenses against oxidative stress. Trends Genetics 1990, 6:363-368, ref. 1) O2- and H2O2 seem to differ in the rate of change with age in both basal levels and inducibility under oxygen stress.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asoh K., Watanabe Y., Mizoguchi H., Mawatari M., Ono M., Kohno K., Kuwano M. Induction of manganese superoxide dismutase by tumor necrosis factor in human breast cancer MCF-7 cell line and its TNF-resistant variant. Biochem Biophys Res Commun. 1989 Jul 31;162(2):794–801. doi: 10.1016/0006-291x(89)92380-2. [DOI] [PubMed] [Google Scholar]
- Burton K. P., McCord J. M., Ghai G. Myocardial alterations due to free-radical generation. Am J Physiol. 1984 Jun;246(6 Pt 2):H776–H783. doi: 10.1152/ajpheart.1984.246.6.H776. [DOI] [PubMed] [Google Scholar]
- Erroi A., Bianchi M., Ghezzi P. The pneumotoxicant paraquat potentiates IL-1 and TNF production by human mononuclear cells. Agents Actions. 1992 May;36(1-2):66–69. doi: 10.1007/BF01991230. [DOI] [PubMed] [Google Scholar]
- Ferrans V. J. Anthracycline cardiotoxicity. Adv Exp Med Biol. 1983;161:519–532. doi: 10.1007/978-1-4684-4472-8_31. [DOI] [PubMed] [Google Scholar]
- Fisher H. K., Humphries M., Bails R. Paraquat poisoning. Recovery from renal and pulmonary damage. Ann Intern Med. 1971 Nov;75(5):731–736. doi: 10.7326/0003-4819-75-5-731. [DOI] [PubMed] [Google Scholar]
- Glass G. A., Gershon D. Enzymatic changes in rat erythrocytes with increasing cell and donor age: loss of superoxide dismutase activity associated with increases in catalytically defective forms. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1245–1253. doi: 10.1016/0006-291x(81)90256-4. [DOI] [PubMed] [Google Scholar]
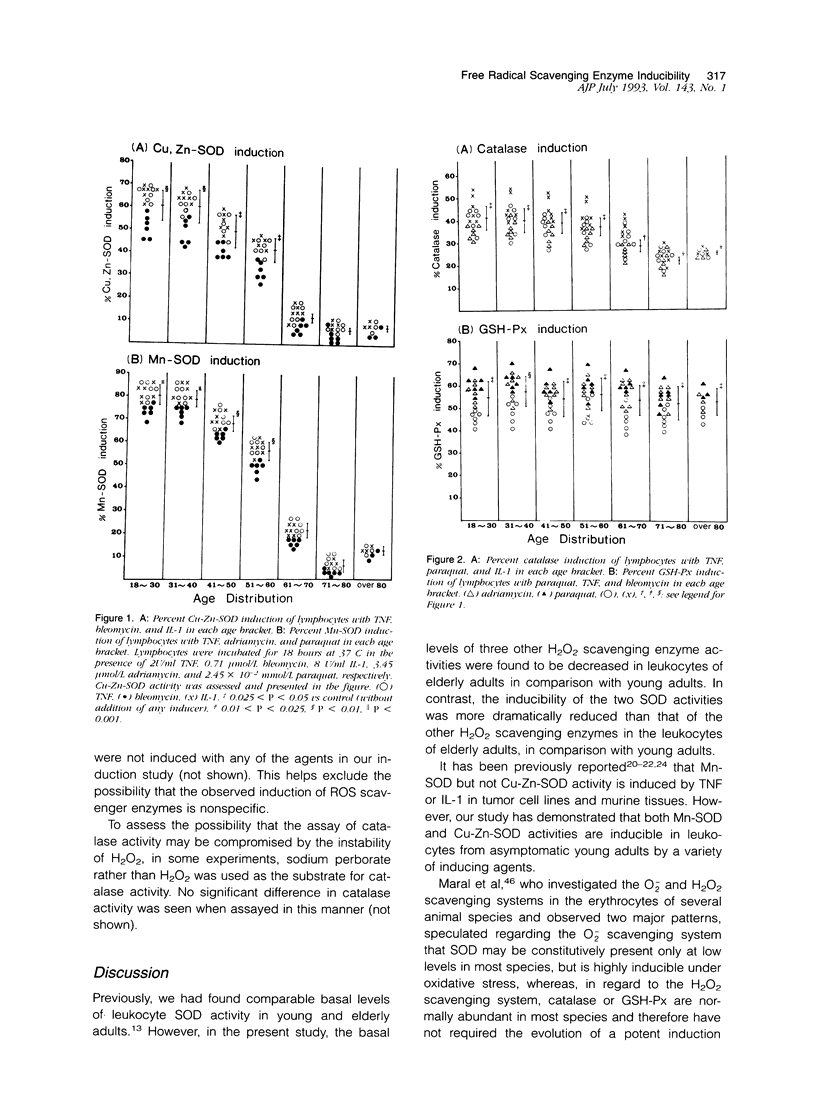
- Goto F., Nakamura S., Goto K., Yoshinaga M. Production of a lymphocyte proliferation potentiating factor by purified polymorphonuclear leucocytes from mice and rabbits. Immunology. 1984 Dec;53(4):683–692. [PMC free article] [PubMed] [Google Scholar]
- Grabensee B., Veltmann G., Mürtz R., Borchard F. Vergiftung durch Paraquat. Dtsch Med Wochenschr. 1971 Mar 19;96(12):498–passim. doi: 10.1055/s-0028-1108282. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. A., Derbin K. S., Hunte-McDonough B., Krauss M. R., Chen K. T., Smith D. M., Epstein L. B. Manganese superoxide dismutase is induced by IFN-gamma in multiple cell types. Synergistic induction by IFN-gamma and tumor necrosis factor or IL-1. J Immunol. 1991 Jul 1;147(1):149–154. [PubMed] [Google Scholar]
- Hasegawa T., Kaneko F., Niwa Y. Changes in lipid peroxide levels and activity of reactive oxygen scavenging enzymes in skin, serum and liver following UVB irradiation in mice. Life Sci. 1992;50(24):1893–1903. doi: 10.1016/0024-3205(92)90550-9. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- Kasama T., Kobayashi K., Sekine F., Negishi M., Ide H., Takahashi T., Niwa Y. Follow-up study of lipid peroxides, superoxide dismutase and glutathione peroxidase in the synovial membrane, serum and liver of young and old mice with collagen-induced arthritis. Life Sci. 1988;43(23):1887–1896. doi: 10.1016/s0024-3205(88)80006-7. [DOI] [PubMed] [Google Scholar]
- Kawai S., Komura J., Asada Y., Niwa Y. Experimental burn-induced changes in lipid peroxide levels, and activity of superoxide dismutase and glutathione peroxidase in skin lesions, serum, and liver of mice. Arch Dermatol Res. 1988;280(3):171–175. doi: 10.1007/BF00456850. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Bush D. M., Kozumbo W. J. Inhibition of tumor promotion by a biomimetic superoxide dismutase. Science. 1983 Jul 1;221(4605):75–77. doi: 10.1126/science.6857269. [DOI] [PubMed] [Google Scholar]
- Kimball R. E., Reddy K., Peirce T. H., Schwartz L. W., Mustafa M. G., Cross C. E. Oxygen toxicity: augmentation of antioxidant defense mechanisms in rat lung. Am J Physiol. 1976 May;230(5):1425–1431. doi: 10.1152/ajplegacy.1976.230.5.1425. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Gallin J. I. Human leukocytic pyrogen induces release of specific granule contents from human neutrophils. J Clin Invest. 1978 May;61(5):1330–1336. doi: 10.1172/JCI109050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Henderson W. R., Gallin J. I. Stimulation of neutrophil oxygen-dependent metabolism by human leukocytic pyrogen. J Clin Invest. 1979 Oct;64(4):996–1002. doi: 10.1172/JCI109566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lefrak E. A., Pitha J., Rosenheim S., Gottlieb J. A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973 Aug;32(2):302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Maral J., Puget K., Michelson A. M. Comparative study of superoxide dismutase, catalase and glutathione peroxidase levels in erythrocytes of different animals. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1525–1535. doi: 10.1016/s0006-291x(77)80151-4. [DOI] [PubMed] [Google Scholar]
- Masuda A., Longo D. L., Kobayashi Y., Appella E., Oppenheim J. J., Matsushima K. Induction of mitochondrial manganese superoxide dismutase by interleukin 1. FASEB J. 1988 Dec;2(15):3087–3091. doi: 10.1096/fasebj.2.15.3263930. [DOI] [PubMed] [Google Scholar]
- Matsubara N., Fuchimoto S., Iwagaki H., Nonaka Y., Kimura T., Kashino H., Edamatsu R., Hiramatsu M., Orita K. The possible involvement of free radical scavenging properties in the actions of cytokines. Res Commun Chem Pathol Pharmacol. 1991 Feb;71(2):239–242. [PubMed] [Google Scholar]
- McCord J. M., Roy R. S. The pathophysiology of superoxide: roles in inflammation and ischemia. Can J Physiol Pharmacol. 1982 Nov;60(11):1346–1352. doi: 10.1139/y82-201. [DOI] [PubMed] [Google Scholar]
- Michelson A. M. Oxygen radicals. Agents Actions Suppl. 1982;11:179–201. [PubMed] [Google Scholar]
- Niwa Y., Ishimoto K., Kanoh T. Induction of superoxide dismutase in leukocytes by paraquat: correlation with age and possible predictor of longevity. Blood. 1990 Aug 15;76(4):835–841. [PubMed] [Google Scholar]
- Niwa Y., Kanoh T., Sakane T., Soh H., Kawai S., Miyachi Y. The ratio of lipidperoxides to superoxide dismutase activity in the skin lesions of patients with severe skin diseases: an accurate prognostic indicator. Life Sci. 1987 Mar 9;40(10):921–927. doi: 10.1016/0024-3205(87)90310-9. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Kasama T., Kawai S., Komura J., Sakane T., Kanoh T., Miyachi Y. The effect of aging on cutaneous lipid peroxide levels and superoxide dismutase activity in guinea pigs and patients with burns. Life Sci. 1988;42(4):351–356. doi: 10.1016/0024-3205(88)90072-0. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Kasama T., Miyachi Y., Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 1989;44(22):1655–1664. doi: 10.1016/0024-3205(89)90482-7. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Miyake S., Sakane T., Shingu M., Yokoyama M. Auto-oxidative damage in Behçet's disease--endothelial cell damage following the elevated oxygen radicals generated by stimulated neutrophils. Clin Exp Immunol. 1982 Jul;49(1):247–255. [PMC free article] [PubMed] [Google Scholar]
- Niwa Y., Sohmiya K. Enhanced neutrophilic functions in mucocutaneous lymph node syndrome, with special reference to the possible role of increased oxygen intermediate generation in the pathogenesis of coronary thromboarteritis. J Pediatr. 1984 Jan;104(1):56–60. doi: 10.1016/s0022-3476(84)80589-2. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Taniguchi S. Phospholipid base exchange in human leukocyte membranes: quantitation and correlation with other phospholipid biosynthetic pathways. Arch Biochem Biophys. 1986 Nov 1;250(2):345–357. doi: 10.1016/0003-9861(86)90736-8. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., Spitz D. R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984;105:457–464. doi: 10.1016/s0076-6879(84)05064-3. [DOI] [PubMed] [Google Scholar]
- Olive C., Levy H. R. The preparation and some properties of crystalline glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides. Biochemistry. 1967 Mar;6(3):730–736. doi: 10.1021/bi00855a012. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Kapanci Y., Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989 Sep 1;170(3):655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch H. D., Clare D. A., Crapo J. D., Fridovich I. Positive correlation between superoxide dismutase and resistance to paraquat toxicity in the green alga Chlorella sorokiniana. Arch Biochem Biophys. 1983 Sep;225(2):640–648. doi: 10.1016/0003-9861(83)90075-9. [DOI] [PubMed] [Google Scholar]
- Reiss U., Gershon D. Comparison of cytoplasmic superoxide dismutase in liver, heart and brain of aging rats and mice. Biochem Biophys Res Commun. 1976 Nov 22;73(2):255–262. doi: 10.1016/0006-291x(76)90701-4. [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Oberley L. W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989 May 15;179(1):8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Stevens J. B., Autor A. P. Induction of superoxide dismutase by oxygen in neonatal rat lung. J Biol Chem. 1977 May 25;252(10):3509–3514. [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Farr S. B., Ames B. N. Bacterial defenses against oxidative stress. Trends Genet. 1990 Nov;6(11):363–368. doi: 10.1016/0168-9525(90)90278-e. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Suzuki T. Nucleotide sequence specificity of DNA cleavage by iron-bleomycin. Alteration on ethidium bromide-, actinomycin-, and distamycin-intercalated DNA. J Biol Chem. 1982 Sep 25;257(18):10544–10546. [PubMed] [Google Scholar]
- Thomson J. F., Nance S. L., Tollaksen S. L. Spectrophotometric assay of catalase with perborate as substrate. Proc Soc Exp Biol Med. 1978 Jan;157(1):33–35. doi: 10.3181/00379727-157-39984. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Lee C. Y., White J. E. Interleukin 1 protects rats against oxygen toxicity. J Appl Physiol (1985) 1991 Aug;71(2):688–697. doi: 10.1152/jappl.1991.71.2.688. [DOI] [PubMed] [Google Scholar]
- WEISS S. B., SMITH S. W., KENNEDY E. P. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958 Mar;231(1):53–64. [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]


